Researchers have developed a new device that promises to dramatically improve how neuroscientists study the mouse brain, which is often the first step toward human studies and drug development.
Featured in Asia Research News 2020 Magazine
Neuroscientists have long dreamed of being able to control brain circuitry remotely as a way to improve long-term animal studies of neurological diseases and complex social behaviours, such as Parkinson's disease and autism. While recent progress has been made in terms of drug delivery and precise control of neurons, most systems have been plagued with shortcomings. Researchers at the Korea Advanced Institute of Science and Technology (KAIST), in collaboration with colleagues at the University of Washington in the U.S., have developed a new device that promises to dramatically improve how neuroscientists study the mouse brain, which is often the first step toward human studies and drug development.
The small 'optofluidic' device can deliver drugs directly into the mouse brain for extended periods of time allowing longterm study of developmental and degenerative disorders. It can also turn targeted brain cells on and off with coloured light, which enables researchers to study specific neural pathways without affecting other parts of the brain. All of this is remotely controlled from a smartphone application.
The remote-controlled, wireless system means animals can move around uninhibited, which is important for studying movement disorders and social behaviour. It also enables individualized wireless drug delivery to nearby animals, and multiple, ongoing studies running side-byside in limited laboratory space. This was unthinkable in the past.
"Having a tool that enables several different studies running in the same room at the same time without direct human interference can help accelerate the pace of research and the process of drug development," says Jae-Woong Jeong, a KAIST biomedical engineer who led the work.
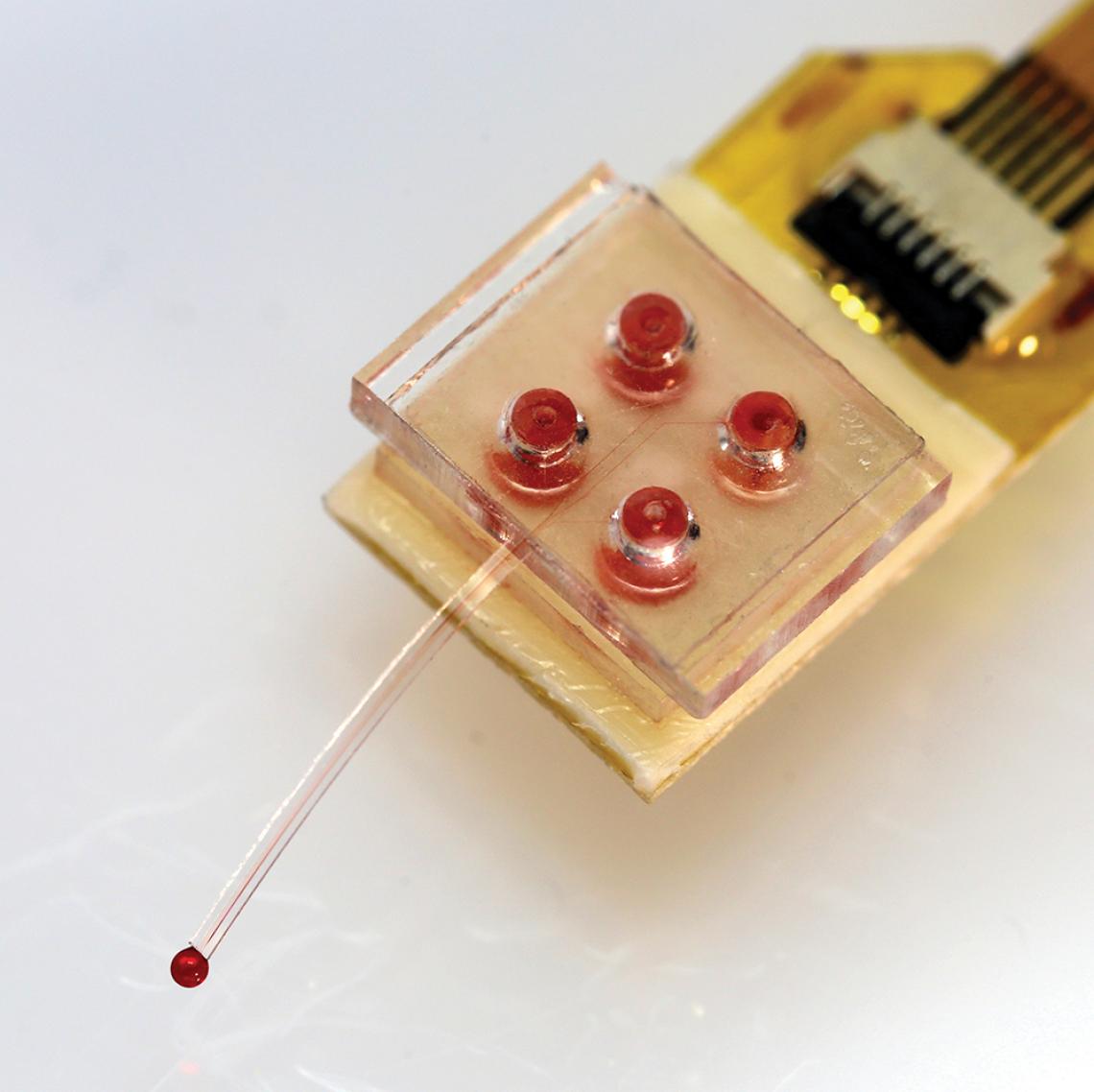
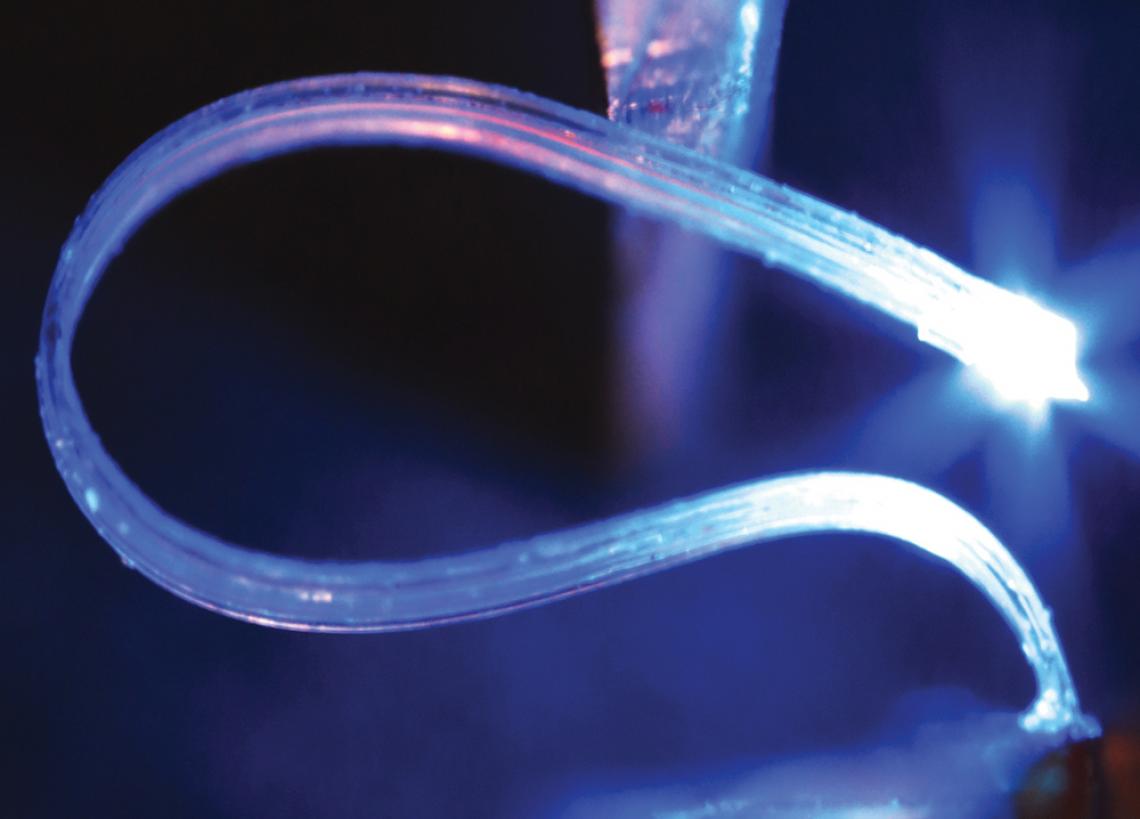
The drug cartridge resembles a Lego brick with four reservoirs that can contain different drugs. An ultrathin microfluidic probe extends into the brain to deliver the drugs. This part is made from a soft, silicon-based organic polymer commonly found in contact lenses. The tip is surrounded by micro-LEDs to also control cells with light.
WHAT'S DIFFERENT ABOUT THIS IMPLANT?
Traditionally, tiny metal tubes, called cannulas, have been used to deliver drugs to the brain, invariably inflaming and damaging brain tissue. To address this, Jeong and his team developed soft, ultrathin, microfluidic probes made from a silicon-based organic polymer commonly found in contact lenses.
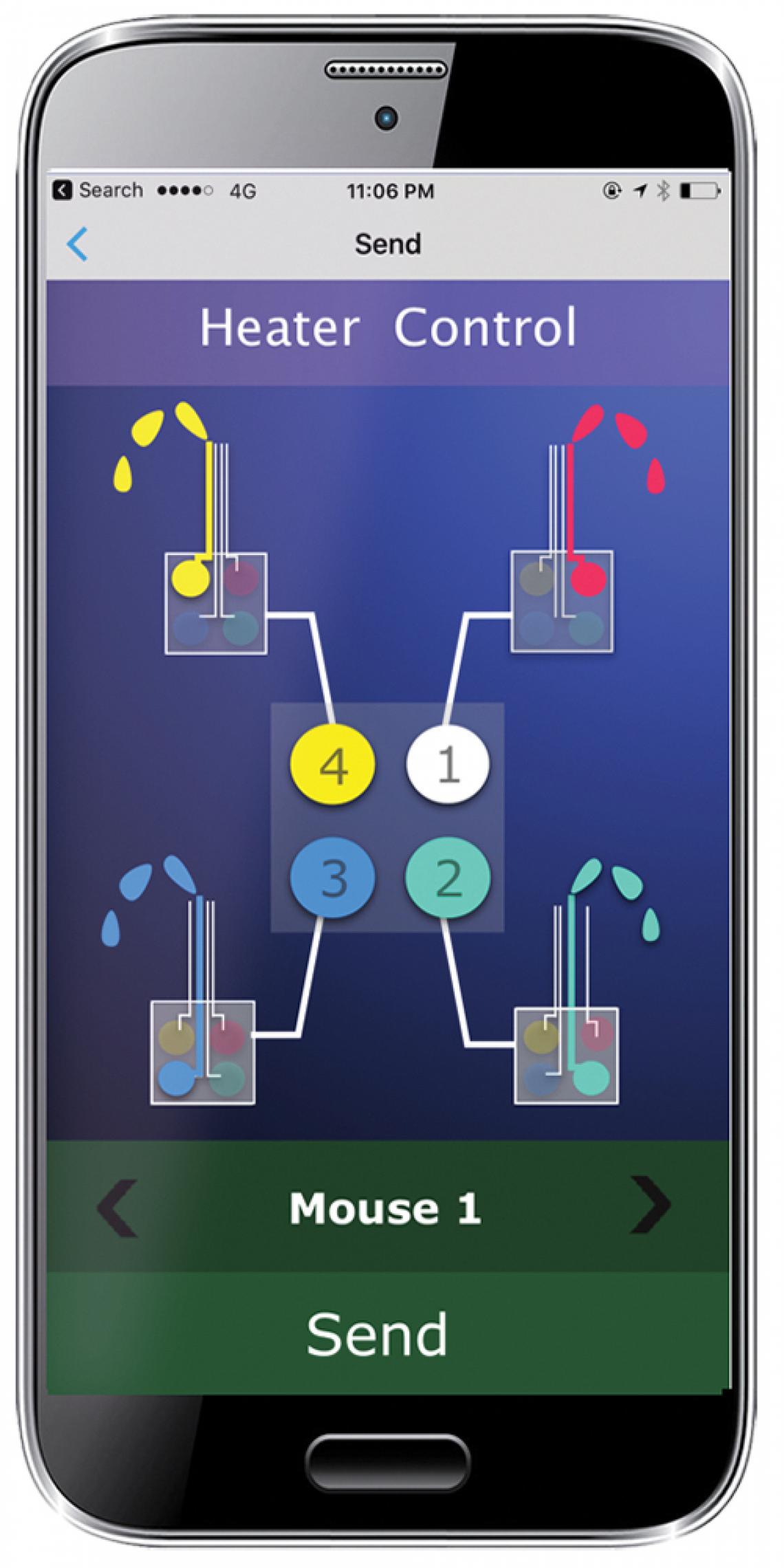
Also, previous wireless drug delivery devices contained a limited supply of drugs and studies usually ended when the drugs ran out. In this new device, drugs are supplied via a detachable cartridge that closely resembles a standard 2 × 2 Lego brick, that sits on top of the mouse’s head. The cartridge’s four reservoirs can be filled with different drugs, which are independently controlled and flow through separate channels within the microfluidic probe when individual pads beneath them are heated. Changing the drug reservoir is easier than changing the ink cartridge on your printer: simply unplug the used cartridge and plug in the new one. This setup allows unlimited and long-term delivery of multiple drugs.
Micro LEDs can turn on or off individual neurons with different colors or wave- lengths of light.
BUT THAT’S NOT ALL
The implant also has the ability to turn targeted nerve cells, or neurons, on and off using light. Optogenetics is a technique used by neuroscientists to control specific neurons by adding light-sensitive ion channels to cell membranes via genetic modifications. Exposing the channels to blue or orange light will either excite or inhibit the neurons. Jeong and his team installed blue and orange micro LEDs around the microfluidic probe, which are also controlled by the smartphone app.
Combining microfluidic drug delivery and optogenetic control of individual neurons into one ‘optofluidic’ device is a major step forward for neuroscience research. But perhaps what makes the new device truly unique and game changing is the integrated, battery-powered Bluetooth Low Energy module and the smartphone app that connects to it. The system allows programmed or manual control of each drug reservoir and each micro LED for multiple devices at the same time.
The device solves many of the problems that have given scientists trouble in the past, making it possible, for the first time, to perform several long-term studies with live animals simultaneously. For example, in their proof-of-concept experiments, researchers used optogenetics to make multiple, nearby, freely moving mice prefer specific locations, and blocked the process in other mice using timely drug delivery. In a separate study, they confirmed the potential longevity of the device by delivering drugs that regulated motor function for at least a month without losing effectiveness.
Developing biomedical devices takes time. The team spent over two years testing more than 20 design iterations, finally arriving at a device that weighs only two grams, does not impede animal behaviour, and works reliably.
“Engineering a reliable plug-n-play interface on such a small scale was a major challenge as there were multiple electrical, mechanical, thermal and material processes that had to be optimized,” explains Raza Qazi, a biomedical engineer in Jeong’s team.
A smartphone app enables wireless, remote-control of multiple experiments at the same time.
BETTER TECHNOLOGY LEADS TO IMPROVED STUDIES
The proof-of-concept experiments are just a taste of what this new device can do. Jeong points out that because of the improved design, neuroscientists will be able to more effectively study mouse models of neurodegenerative disorders that develop over long periods of time, such as Parkinson’s disease and Alzheimer’s disease. Long-term testing of a drug or combination of drugs without needing to switch animals or perform additional implant surgeries will likely yield more accurate assessments of the effects drugs have on chronic diseases.
Engineering a reliable plug-nplay interface on such a small scale was a major challenge.
For some neuroscientists, the largest benefit will be the wireless design allowing complete freedom of movement, which can improve the study of complex social behaviour. This is particularly necessary when testing how drugs affect interactions in animal models of mental and social disorders, such as depression, autism spectrum disorders, and schizophrenia.
Drug development is a slow process that goes through rigorous study in animals before human clinical trials. The wireless technology and easy control of individualized drug delivery to numerous animals can also enable the simultaneous coordination of multiple experiments, thus increasing research throughput and speeding up the process. Engineering a reliable plug-nplay interface on such a small scale was a major challenge. Micro LEDs can turn on or off individual neurons with different colors or wavelengths of light.
Further information
Professor Jae-Woong Jeong | E-mail: [email protected]
School of Electrical Engineering
Korea Advanced Institute of Science and Technology
Read this story in the Asia Research News 2020 magazine.
The many ways we can tell your research story. Find out more from our Content services page.
When drugs run out, the cartridge can be easily swapped for a full one, enabling longer studies than in the past.