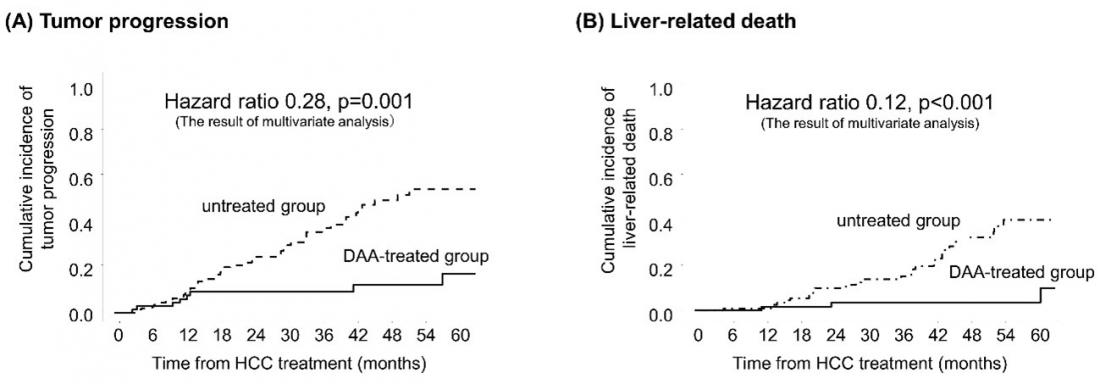
Cumulative incidence of tumor progression (A) and liver-related mortality (B) by Kaplan-Meier analysis. Tumor progression was defined as when HCC progresses to multiple nodules in the liver, portal invasion or metastasizes to other parts of the body, bringing the disease from early- to advanced-stage HCC, according to the Barcelona Clinic Liver Cancer staging system. Cumulative incidence of tumor progression (A) and liver-related mortality (B) by Kaplan-Meier analysis. Tumor progression was defined as when HCC progresses to multiple nodules in the liver, portal invasion or metastasizes to other parts of the body, bringing the disease from early- to advanced-stage HCC, according to the Barcelona Clinic Liver Cancer staging system.
OSAKA, Japan — In a new cohort study of patients with hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC), a disease with a high recurrence rate, researchers at the Osaka City University Graduate School of Medicine reported that after receiving cancer treatment, the oral administration of direct-acting antivirals (DAA) reduces the risk of tumor progression following recurrence of the liver disease. The findings were published in the Journal of Viral Hepatitis.
Led by Norifumi Kawada, professor of the Department of Hepatology, the study investigated the effect eliminating HCV had on tumor progression* of early-stage HCC. “DAA therapy is effective at eradicating the hepatitis C virus, a major risk factor for HCC” says Professor Kawada. “While it is deemed low or inconclusive whether DAA therapy helps prevent HCC recurrence, little is known about how the antiviral therapy affects progression of the liver disease after cancer treatment.”
165 patients with early-stage HCC, who were also receiving curative HCC treatment, enrolled in the study. After treatment, 72 patients received DAA therapy while the remaining 93 did not. The team recorded 96 HCC recurrences with approximately 75% of them at an early stage according to the Barcelona Clinic Liver Cancer staging system. To account for bias inherent in a single-center retrospective study, a multivariate adjusted time-varying Cox regression analysis showed a 72% reduction in tumor progression in the DAA-treated group and an 88% reduction in risk of death from HCC. Also, the frequency of cancer treatments given before the cancer progressed saw a 59% decrease, from 0.83 treatments per year in patients who did not receive DAA therapy to 0.24 treatments per year in patients who did.
“Usually, cancer cells grow over long periods of time before they can be detected as a tumor,” states first author Hiroko Ikenaga. “Our study showed that eliminating the hepatitis C virus with DAA suppresses tumor progression, which we suggest contributes to overall patient survival.”
HCV infection affects 71 million people worldwide and accounts for about 65% of the causes of liver cancer in Japan. While the team will continue to investigate issues like the extent to which liver cirrhosis and function improve after the antiviral treatment, as supporting author and Lecturer Sawako Uchida-Kobayashi puts it, “the suppressing effect of DAA therapy on cancer progression revealed by our study gives us hope to improve the overall quality of life for people with HCC.”
*Tumor progression was defined as when HCC progresses to more than 4 nodules in the liver, portal invasion or extrahepatic metastasis.
###
We are Osaka City University - the oldest research university in Osaka. With 9 undergraduate faculties and 11 graduate schools all dedicated to making urban life better, energy cleaner, and people healthier and happier, we have won numerous awards and have produced 2 Nobel laureates. For more information, please visit our website at https://www.osaka-cu.ac.jp/en