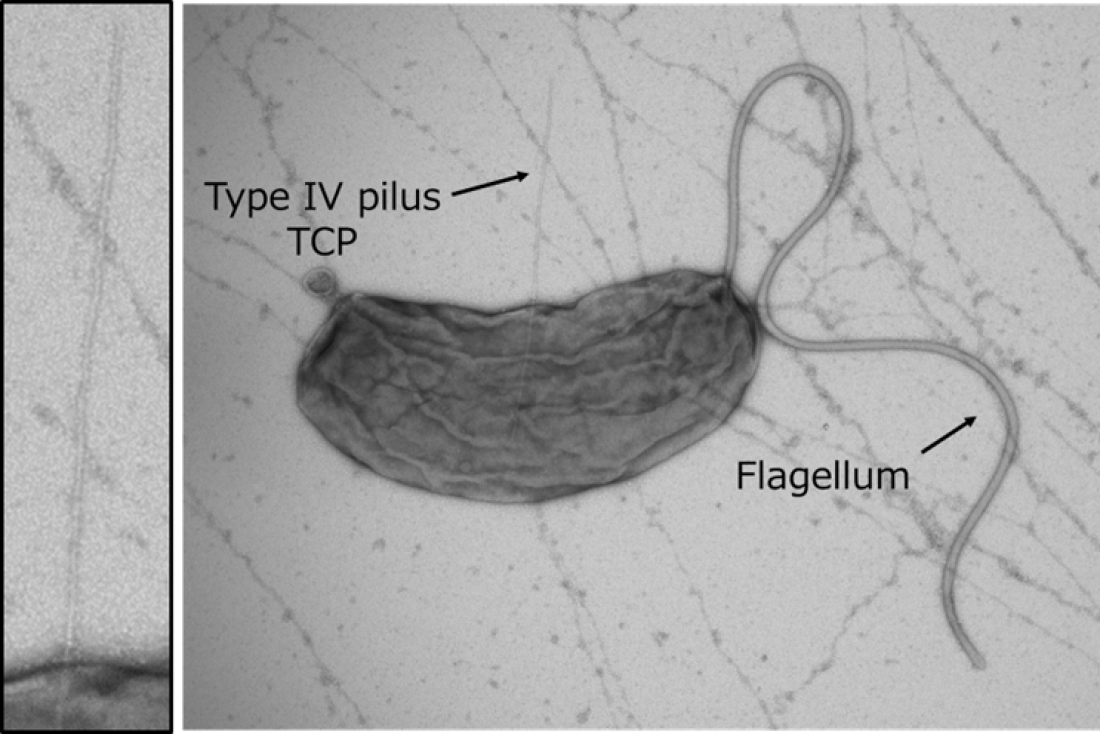
Fig.1
Transmission electron micrograph of Vibrio cholerae strain O395. Left: Close-up view of type IV pilus TCP.
Researchers from Osaka University identify the mechanism by which Vibrio cholerae colonization factor TcpF is secreted extracellularly by the Toxin-coregulated pilus
Osaka, Japan – Bacterial infectious diseases are still a huge contributor to global disease burden and with antibiotic resistance on the rise worldwide there is an urgent need for novel treatment strategies against bacteria. One of the most devastating bacterial infections is Cholera, caused by the bacteria Vibrio cholerae, which has been in its 7th ongoing pandemic since 1961. Now, a research group led by Osaka University in Japan has shed light on a specific protein interaction that has the potential to be a novel target in Cholera treatment.
Cholera is characterized by severe diarrhea that can be fatal within hours at its worst. One of the most important steps in the infection process of V. cholerae is for the bacterium to colonize the human intestine by secreting a colonization factor called TcpF, though the exact mechanism behind this secretion remained elusive. Now, in a study that will be published soon in Science Advances, researchers used X-ray crystallography, physicochemical analyses, and structural modeling to reveal exactly how V. cholerae secretes TcpF.
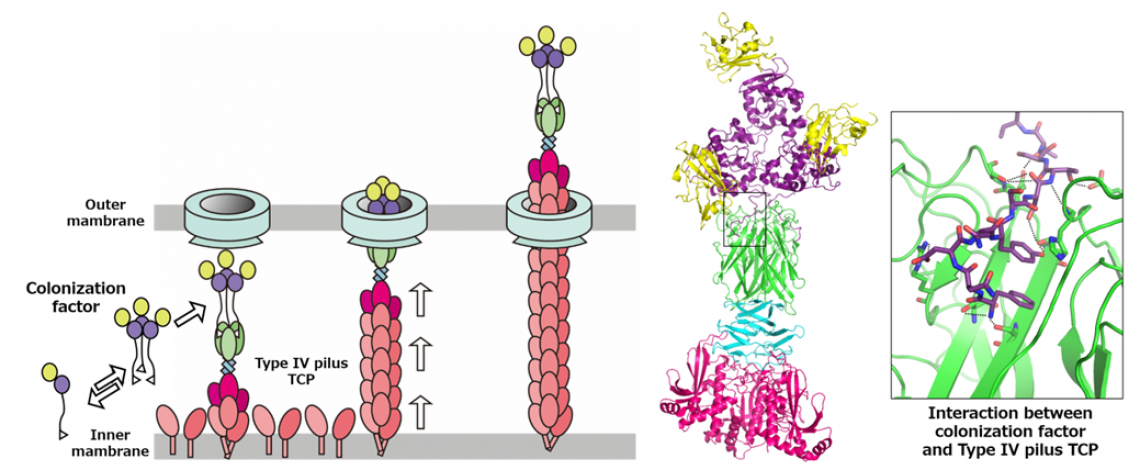
“It was known that the Toxin-coregulated pilus (TCP), a type 4 pilus system, played a crucial role in TcpF secretion, but the exact interaction between the two was unclear” states Hiroya Oki, lead author of the study. Pili are filament-like structures on the surface of bacterial cells that can have a multitude of functions. The V. cholerae TCP is composed primarily of numerous TcpA subunits, with an initial minor subunit comprising a TcpB trimer attaching to the “top” of the pilus to facilitate its assembly. The group studied the interaction of TcpF with TcpA and B and created models based on the results.
“We observed that TcpF trimerized into a flower-like unit to bind to the TcpB trimer at the end of the pilus,” explains Shota Nakamura, senior author of the study. “Importantly, we identified separate conserved domains that are vital for binding of TcpF to TcpB and TcpF trimerization, both of which are required for V. cholerae colonization.
When considering their findings in context with other published works, the group hypothesized a model of secretion where TCP carries TcpB-bound TcpF out of the cell, after which TcpF dissociates from the pilus and moves freely in the human intestine, initiating the early stages of V. cholerae colonization. TCP then retracts back into the bacterial cell to repeat the process.
Given the growing resistance to antibiotics, findings like these that clarify the molecular details of infection can be highly valuable for designing new antibacterial drugs. The development of an anti-adhesive agent that selectively inhibits the interaction between the TcpF colonization factor and the TCP secretion system could provide a novel treatment strategy to combat Cholera.
###
The article, “Structural basis for the toxin-coregulated pilus-dependent secretion of Vibrio cholera colonization factor,” will be published soon in Science Advances at DOI: https://doi.org/10.1126/sciadv.abo3013.
Fig.2
Left: Model of the Type IV pilus system transporting the soluble colonization factor. Right: Crystal structure of TCP in complex with the colonization factor.
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en