While sapphire and quartz are oxide crystals used in a wide range of industrial applications, the atomic-scale structures of these materials are not well understood. The major chemical components of sapphire and quartz are aluminum oxide and silicon dioxide, respectively. These components have a high affinity for water, which affects the chemical reactivity of the crystals. Thus, a thorough knowledge of the water-binding properties of these oxides is important for further innovative applications.
To date, traditional microscopic methods have only provided insights into the two-dimensional topography of their surfaces. Now, a research team led by Keisuke Miyazawa from the NanoLSI at Kanazawa University has developed three-dimensional (3D) microscopy technique for a detailed study of the interaction of the surfaces of these materials with water.
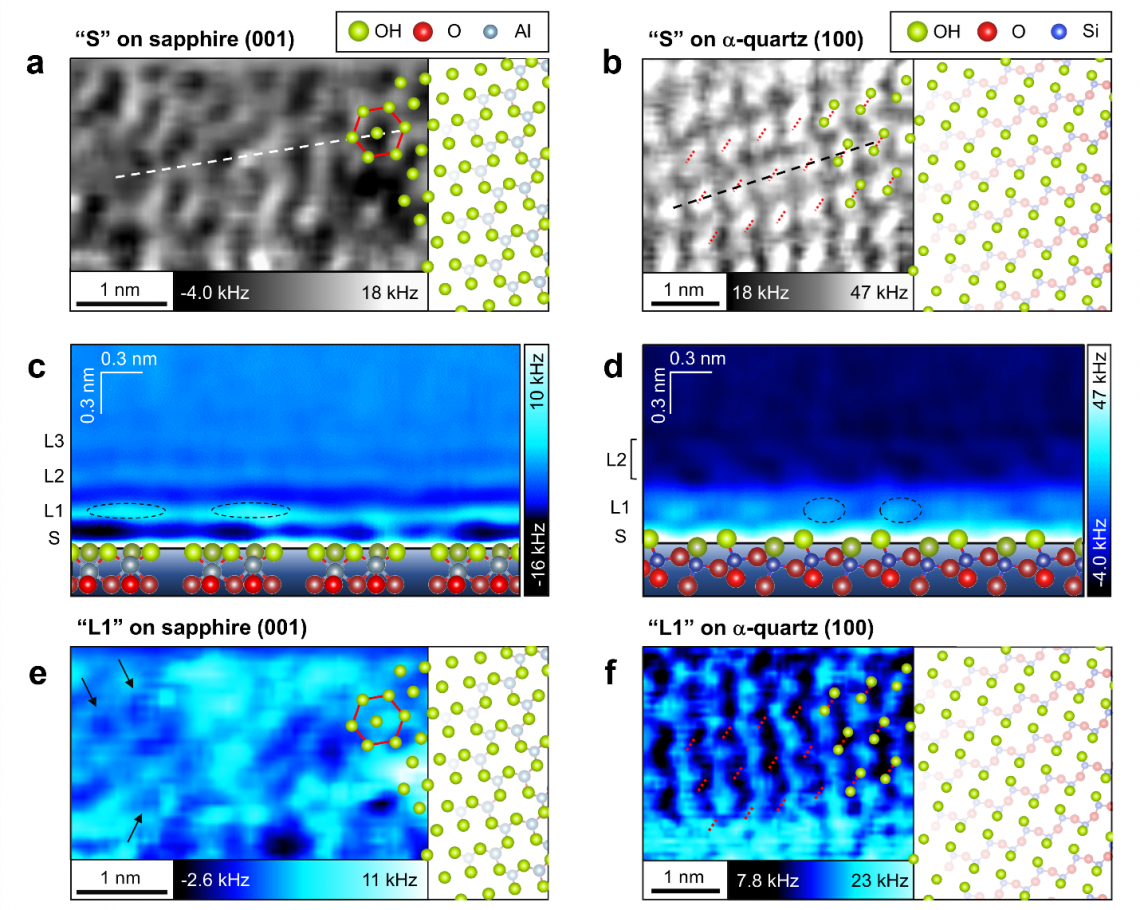
The team started by looking at the surface structures and its hydration structures of sapphire and α-quartz in water. For this, they used an advanced form of microscopy known as 3D atomic force microscopy (3D-AFM). Oxide crystals usually have hydroxyl (OH) groups, which are the main “water-binding” molecules, closely linked with the oxides. Hence, the team studied the OH groups and its hydration structures on both crystals when immersed in water. They found that the hydration layer on sapphire was not uniform because of the nonuniform local distributions of the surface OH groups. On the other hand, the hydration layer on α-quartz was uniform because of the atomically flat distributions of the surface OH groups.
When the interaction force of these oxides for water was subsequently measured, it was found that a greater force was required to break the water-crystal bonds in sapphire than in α-quartz. Lastly, it was also discovered that this affinity was much higher in regions where the oxides were in close proximity to the OH groups.
This study showed that the hydration structures of oxides are dependent on the location and density of OH groups, in addition to the hydrogen bonding (the chemical bond used to bind to water) strength of the OH groups. What's more, it was successfully shown here that 3D-AFM can be used in unraveling the interaction of water with several surfaces, a potential avenue for understanding solid-liquid interactions better. “This study contributes to the application of 3D-AFM in exploring atomic scale hydration structures on various surfaces, and hence, to a wide range of solid–liquid interfacial research fields,” conclude the researchers.
Background
3D atomic force microscopy (3D-AFM): AFM is an advanced form of microscopy wherein a sharp tip is mounted on a cantilever and follows the surface of a molecule. As it does so, the tip emits signals based on its movement, which helps identify the topography of the molecule. However, understanding the deeper structures of molecules requires a three-dimensional overview of their surfaces. Thus, researchers used a more advanced version of AFM in this study, which captured the structure of hydrated crystals in 3D.
Top. Atomic-scale structures of the (a) sapphire and (b) α-quartz surfaces in water. (green = OH groups and red and blue = oxide groups).
Middle. Vertical cross-sections of hydration structures on the (c) sapphire and (d) α-quartz surfaces.
Bottom. 1st hydration layers on the (e) sapphire and (f) α-quartz surfaces.