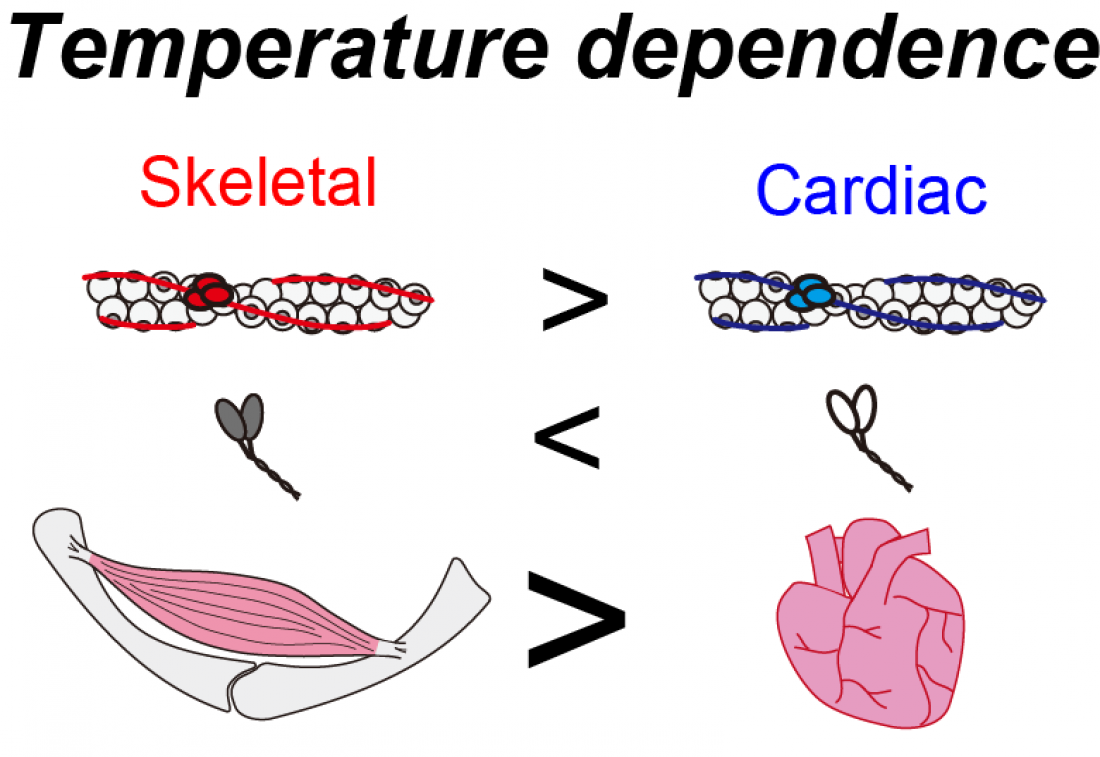
Contractile proteins of skeletal and cardiac muscle have different temperature dependence. Although skeletal myosin is less dependent on temperature than cardiac myosin (middle), skeletal thin filaments are more dependent on temperature than cardiac thin filaments (top). Overall, skeletal muscle is more sensitive to heating than the heart (bottom).
Research out of Osaka University finds that even a tiny increase in skeletal muscle temperature can rapidly activate contractile proteins and improve muscle performance, more so than in cardiac muscle
Osaka, Japan – Everybody knows the importance of warming up your muscles before a workout. But what is actually going on when we warm our muscles up, and are all muscles the same? You might be surprised to find out that the science behind this routine activity hasn’t always been clear.
Now, in a study recently published in the Journal of General Physiology, a multi-institutional research team, led by Osaka University, The Jikei University School of Medicine and National Institutes for Quantum Science and Technology, has revealed how heating affects the contraction of different muscles, and how this might benefit populations in need of improved exercise performance.
Skeletal muscle contracts in response to electrical signals from the nervous system, which activate proteins in muscle cells and allow us to move. The team previously explored how cardiac muscle contractions are affected by temperature, determining that our heart can contract efficiently within the body temperature range.
Next, using muscle proteins and advanced microscopy, the research team wanted to determine how temperature affects skeletal muscle: do skeletal muscles have similar temperature sensitivity, or are they different from the muscles of the heart?
The research team found that some of the proteins in the muscle cells act as a temperature sensor, and that heating affects skeletal and cardiac contractile systems differently.
“Our findings point to differences in the temperature sensitivity of proteins responsible for contraction in skeletal vs. cardiac muscles,” says co-lead author Kotaro Oyama. “Basically, the skeletal muscle that moves our body around is more sensitive to heating than the heart.”
The physiological significance of these findings will become clear when the functional difference between skeletal and cardiac muscle is considered. While skeletal muscle only generates a certain amount of force when required, the heart is meant to beat continuously.
“The higher temperature dependence of skeletal muscle may allow it to contract relatively quickly upon warming up, even from slight warming due to light movement or exercise. This means that the muscle can save energy and rest when not needed. In contrast, the lower temperature sensitivity of the heart may be beneficial for maintaining a continuous beat, regardless of temperature,” explains co-lead author Shuya Ishii.
This study provides new insights into how, at the protein level, warm-up before exercise enhances muscle performance. The discovery that some muscle proteins act as a temperature sensor may lead to a new hyperthermia strategy, in which skeletal muscle performance is improved by warming up the muscle. Incorporating appropriate warm-up routines into the daily lives of individuals, particularly the elderly population, could improve their muscle and exercise performance, thereby reducing the risk of injury and helping to maintain their independence.
###
The article, “Myosin and tropomyosin-troponin complementarily regulate thermal activation of muscles,” has been published in Journal of General Physiologyat DOI: https://doi.org/10.1085/jgp.202313414
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en
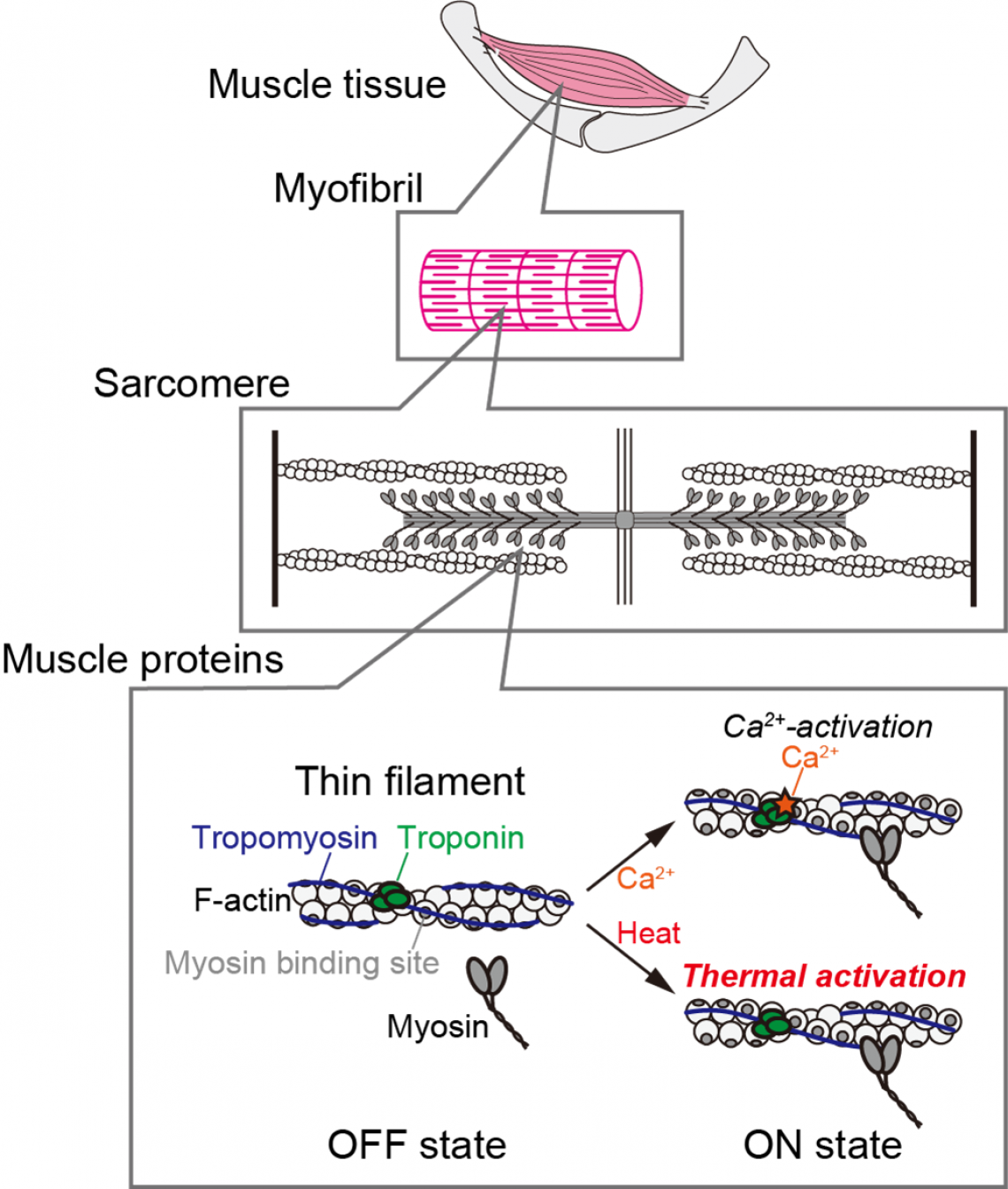
Hierarchical structure of muscle and activation of thin filaments. In both skeletal and cardiac muscles under a relaxed state, a low intracellular calcium ion concentration is maintained and the tropomyosin-troponin complex suppresses interaction between molecular motor myosin and actin filament (F-actin); this is termed the OFF state. Upon an increase in the intracellular calcium ion concentration, or by calcium signaling, the binding of calcium ion to troponin leads to the displacement of tropomyosin. The conformational change of the thin filaments allows myosin to interact with actin, and active force is generated; this is termed the ON state. Heat works as “thermal signaling,” inducing an ON state even in the absence of calcium ions.