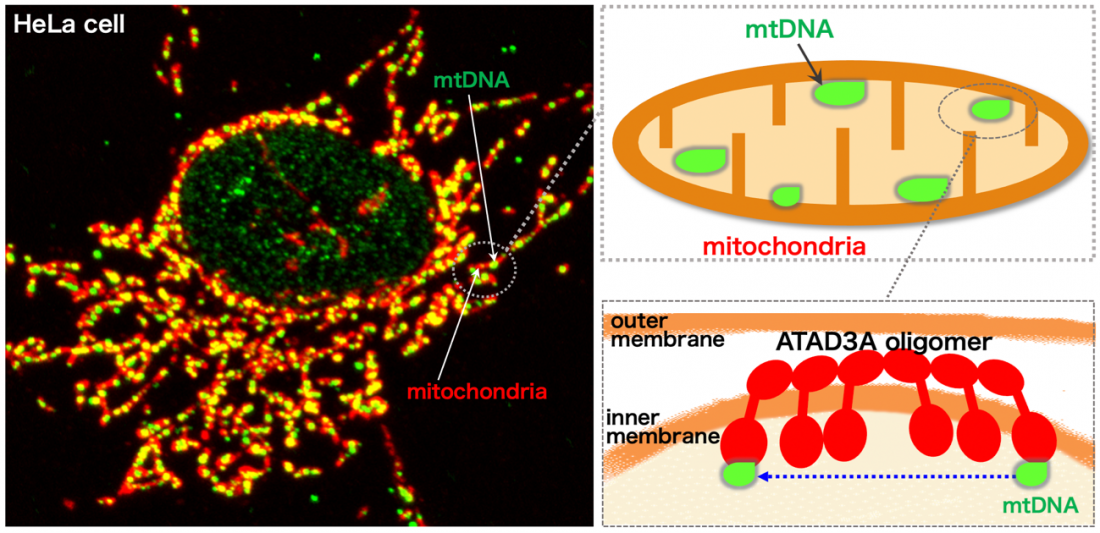
Fig. 1
Inner-membrane AAA-ATPase ATAD3A drives mtDNA
Researchers led by Osaka University find that a molecule called ATAD3A is essential for the movement of genetic material inside mitochondria, affecting energy production
Osaka, Japan – Mitochondria, famously known as the powerhouse of the cell, are important cellular structures that are vital for their role of generating energy. Mitochondria are “dynamic”, meaning they constantly fuse together and split apart. They contain a small amount of genetic information known as mitochondrial DNA (mtDNA). The mtDNA, organized into dot-like structures called “nucleoids”, also moves around inside the mitochondria. The method of distribution of mtDNA remained unclear, but now a research team led by Osaka University has identified a molecule known as ATAD3A that is essential for nucleoid movement and could be a potential therapeutic candidate for mitochondrial diseases.
The team had previously shown that the movement of the nucleoids is linked to the fission of mitochondria. However, the mechanisms and function of this movement were unclear. The researchers therefore investigated the role of ATAD3A in nucleoid movement because of its previously established links to nucleoid formation.
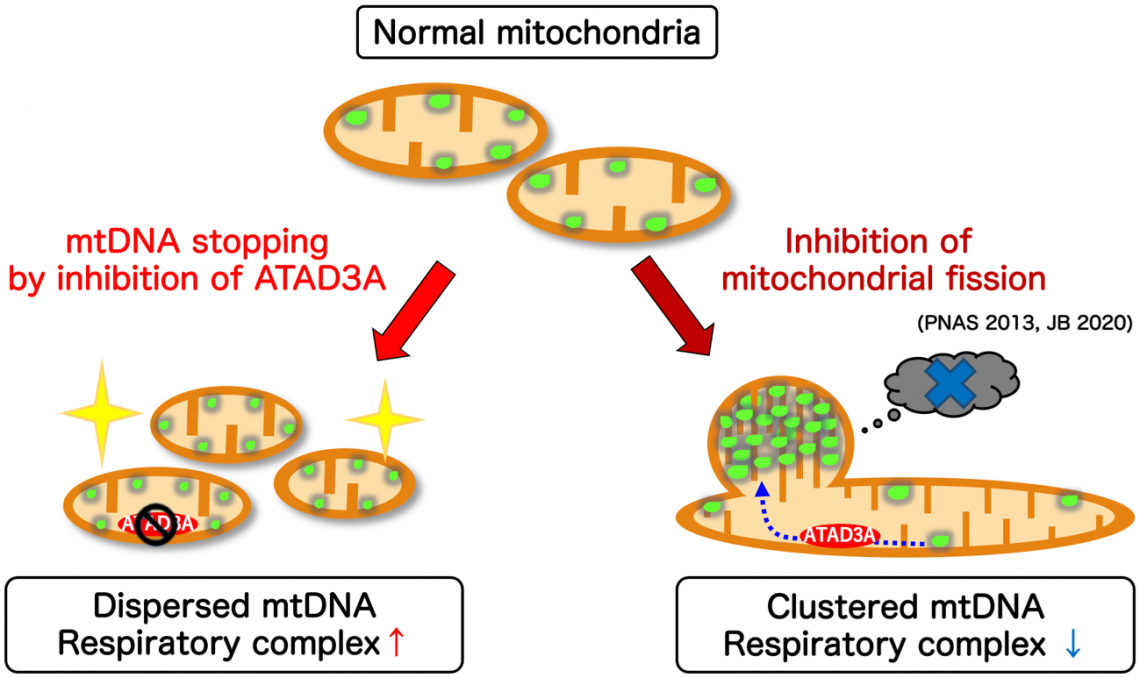
In a study published recently in the Proceedings of the National Academy of Sciences, the researchers showed that ATAD3A, which is anchored to the inner mitochondrial membrane, mediated the interaction of mtDNA nucleoids (present inside the mitochondria) with factors involved in mitochondrial fission (present on the outer mitochondrial membrane). They demonstrated that ATAD3A was essential for the active movement of mtDNA nucleoids within the mitochondria—a process called nucleoid “trafficking”—and that nucleoids were abnormally clustered in cells that lack mitochondrial fission. Together, mitochondrial fission and nucleoid trafficking determine the size, number, and distribution of the nucleoids within the mitochondria.
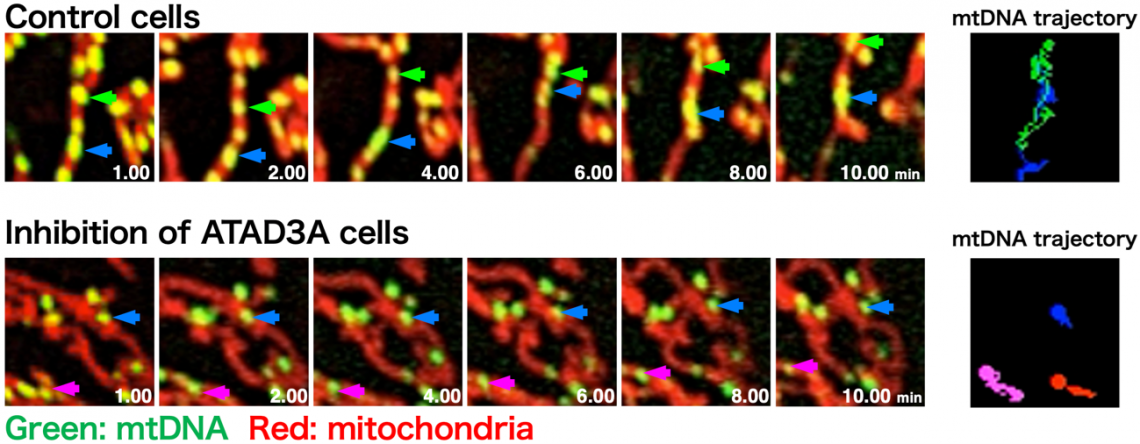
Fig. 2
Suppression of ATAD3A stops mtDNA movement: time-lapse observation using spinning-disc confocal microscopy was used to capture the movement of mtDNA (images are still images at each timepoint). Arrowheads indicate randomly selected and tracked mtDNA in a part of the live-cell imaging (right: mtDNA trajectory). When ATAD3A expression is suppressed, mtDNA hardly moves.
The distribution of nucleoids throughout the mitochondrial network activates expression of the mtDNA and increases formation of the “respiratory chain complex”, which is a group of several proteins essential for energy production within cells, and the correct distribution of the mtDNA nucleoids is key for efficient energy production. “Regulation of nucleoid dynamics is crucial for the maintenance of respiratory chain complexes on the mitochondrial inner membrane,” explains lead author Takaya Ishihara, “and this is the first report of the role of ATAD3A in mitochondrial nucleoid dynamics.”
The development of techniques to alter mtDNA movement may help regulate mitochondrial function in the future. “Nucleoid trafficking may represent a new therapeutic target to prevent mitochondrial dysfunction in various human diseases,” explains senior author Naotada Ishihara.
Therefore, not only does this work increase our knowledge of the regulatory processes in mitochondria but it also provides scope for developing future therapies against abnormal mitochondrial functioning.
###
The article, “Mitochondrial nucleoid trafficking regulated by the inner-membrane AAA-ATPase ATAD3A modulates respiratory complex formation”, was published in PNAS at DOI: https://doi.org/10.1073/pnas.2210730119
Fig. 3
Inner-membrane AAA-ATPase ATAD3A regulates respiratory complex formation
by modulating mtDNA dynamics.
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en