Tokyo, Japan – From lithium-ion batteries to next-generation superconductors, the functionality of many modern, advanced technologies depends on the physical property known as intercalation. Unfortunately, it's difficult to identify in advance which of the many possible intercalated materials are stable, which necessitates a lot of trial-and-error lab work in product development.
Now, in a study recently published in ACS Physical Chemistry Au , researchers from the Institute of Industrial Science, The University of Tokyo, and collaborating partners have devised a straightforward equation that correctly predicts the stability of intercalated materials. The systematic design guidelines enabled by this work will speed up the development of upcoming high-performance electronics and energy-storage devices.
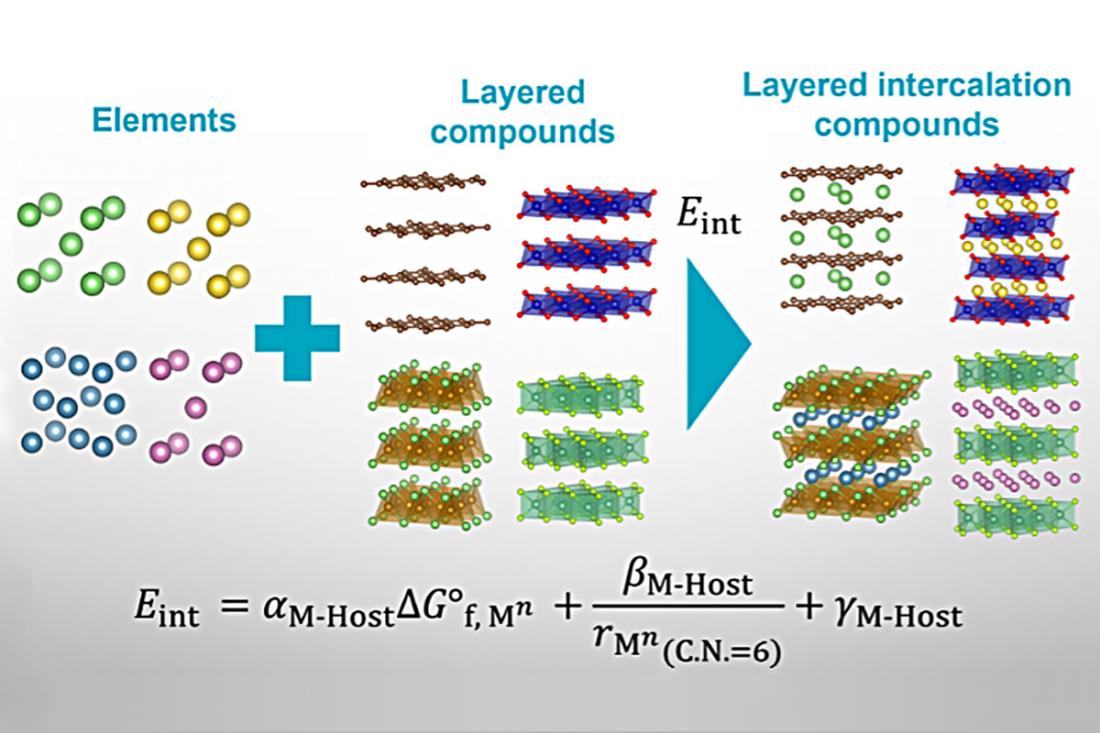
To appreciate the research team’s achievement, we need to understand the context of this research. Intercalation is the reversible insertion of guests (atoms or molecules) into hosts (for example, 2D-layered materials). The purpose of intercalation is commonly to modify the host’s properties or structure for improved device performance, as seen in, for example, commercial lithium-ion batteries. Although many synthetic methods are available for preparing intercalated materials, researchers have had no reliable means of predicting which host–guest combinations are stable. Therefore, much lab work has been needed to devise new intercalated materials for imparting next-generation device functionalities. Minimizing this lab work by proposing a straightforward predictive tool for host–guest stability was the goal of the research team's study.
"We are the first to develop accurate predictive tools for host–guest intercalation energies, and the stability of intercalated compounds," explains Naoto Kawaguchi, lead author of the study. "Our analysis, based on a database of 9,000 compounds, uses straightforward principles from undergraduate first-year chemistry."
A particular highlight of the work is that only two guest properties and eight host-derived descriptors were necessary for the researchers' energy and stability calculations. In other words, initial ‘best guesses’ weren't necessary; only the underlying physics of the host–guest systems. Furthermore, the researchers validated their model against nearly 200 sets of regression coefficients.
"We're excited because our regression model formulation is straightforward and physically reasonable," says Teruyasu Mizoguchi, senior author. "Other computational models in the literature lack a physical basis or validation against unknown intercalated compounds."
This work is an important step forward in minimizing the laborious lab work that's typically required to prepare intercalated materials. Given that many current and upcoming energy storage and electronic devices depend on such materials, the time and expense required for corresponding research and development will be minimized. Consequently, products with advanced functionalities will reach the market faster than what has been previously possible.
###
The article, "Unraveling the stability of layered intercalation compounds through first principles calculations: Establishing a linear free energy relationship with aqueous ions," was published in ACS Physical Chemistry Au at DOI:10.1021/acsphyschemau.3c00063.
About Institute of Industrial Science, The University of Tokyo
The Institute of Industrial Science, The University of Tokyo (UTokyo-IIS) is one of the largest university-attached research institutes in Japan. UTokyo-IIS is comprised of over 120 research laboratories—each headed by a faculty member—and has over 1,200 members (approximately 400 staff and 800 students) actively engaged in education and research. Its activities cover almost all areas of engineering. Since its foundation in 1949, UTokyo-IIS has worked to bridge the huge gaps that exist between academic disciplines and real-world applications.