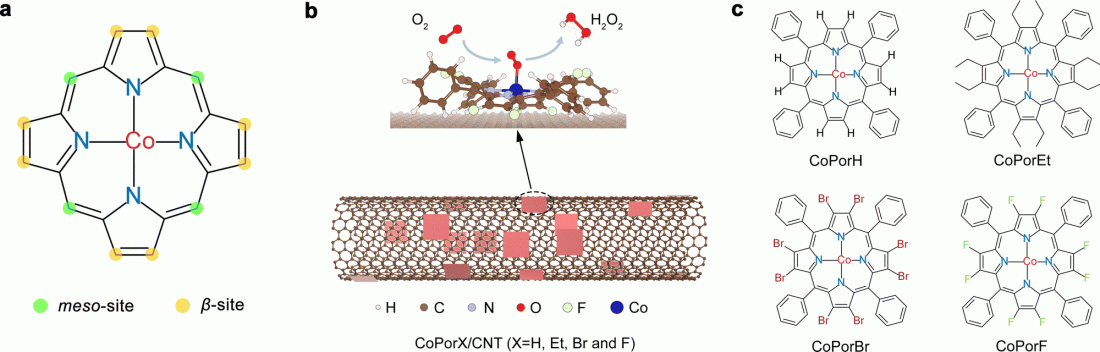
The designed structure of heterogenous molecular catalysts from cobalt porphyrins with various substituents.
Hydrogen peroxide (H2O2) is an industrially important chemical with versatile applications. However, the traditional method used to produce H2O2 is energy intensive and produces significant emissions.
As a means to achieve sustainable development, scientists have sought to synthesize H2O2 electrochemically. This can be done via the oxygen reduction reactions catalyzed by a single cobalt-nitrogen-carbon (Co-N-C) catalyst. But tailoring the precise catalyst atomic structure has been a struggle.
Now, an international group of researchers has theoretically designed a Co-N-C catalyst with unique structures for high-performing electrochemical H2O2 synthesis. The group successfully verified their prediction after experimentally implementing the synthesis, analyzing its characterizations, and carrying-out catalytic tests.
Details of their findings were published in the journal Energy & Environmental Science.
"To date, the search for a catalyst has been carried out based on exhaustive trial-and-error experiments," says Hao Li, associate professor at Tohoku University's Advanced Institute for Materials Research (WPI-AIMR) and corresponding author of the paper. "Our discovery showed that theory-guided research can provide precise design guidelines for catalytic experiments, saving time, money, and human resources."
The group comprised researchers from Japan, Australia, Canada, and China. In particular, Li and his colleague and fellow corresponding author, Li Wei, received support from the University of Sydney under the international SDG-Collaboration Program, a program that promotes international collaboration on SDG-related research between the University of Sydney and other universities.
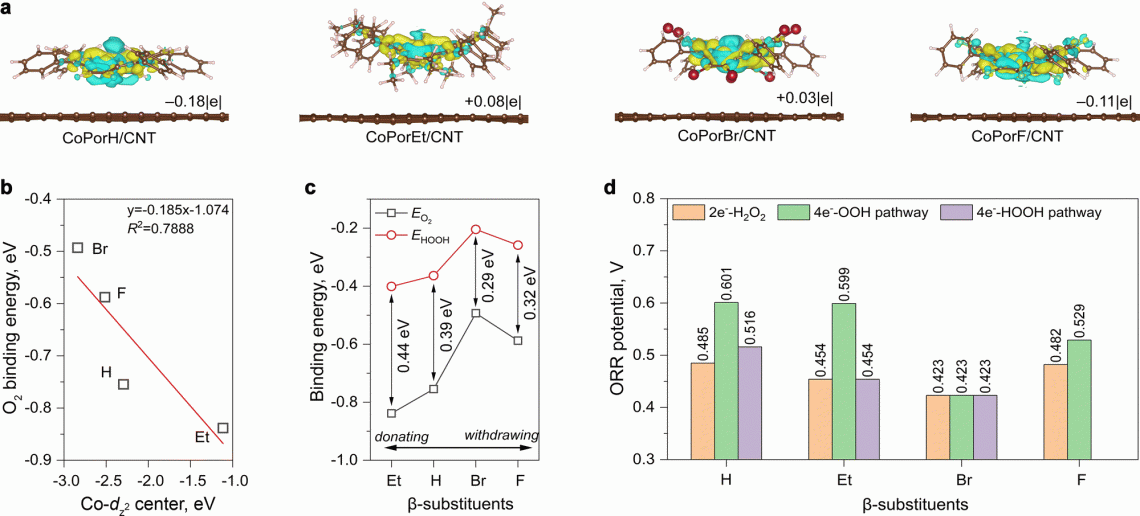
Theoretical analyses and predictions of the catalysts' performance for electrochemical H202 synthesis.
To address the stumbling block of H2O2, the researchers constructed a heterogenous molecular catalyst from cobalt porphyrins absorbed on a carbon nanotube substrate. Their initial calculations suggested that porphyrin β-substituents and the carbon substrate could synergistically modulate Co properties and catalytic activity.
They further predicted the optimality of an octafluoro-substituted catalyst and validated their predictions through experiments, with it exhibiting >94% H2O2 selectivity and a high turnover frequency of 3.51 per second at an overpotential of 200 millivolts in an acid electrolyte. Furthermore, it reached a maximum H2O2 productivity of 10.76 molH2O2 gcat−1 h−1 in a two-electrode electrolyzer, delivering pure H2O2 solutions that can be used directly for water treatment and chemical production.
Looking ahead, Li hopes to design further Co-N-C catalysts. "By tuning the type of metal center and their coordination environments, and through extensive performance and stability testing, we hope to uncover more metal-N-C for use in various electrocatalysis.
Experimental verifications and H2O2 synthesis performance of the four designed molecular Co-N-C catalysts.