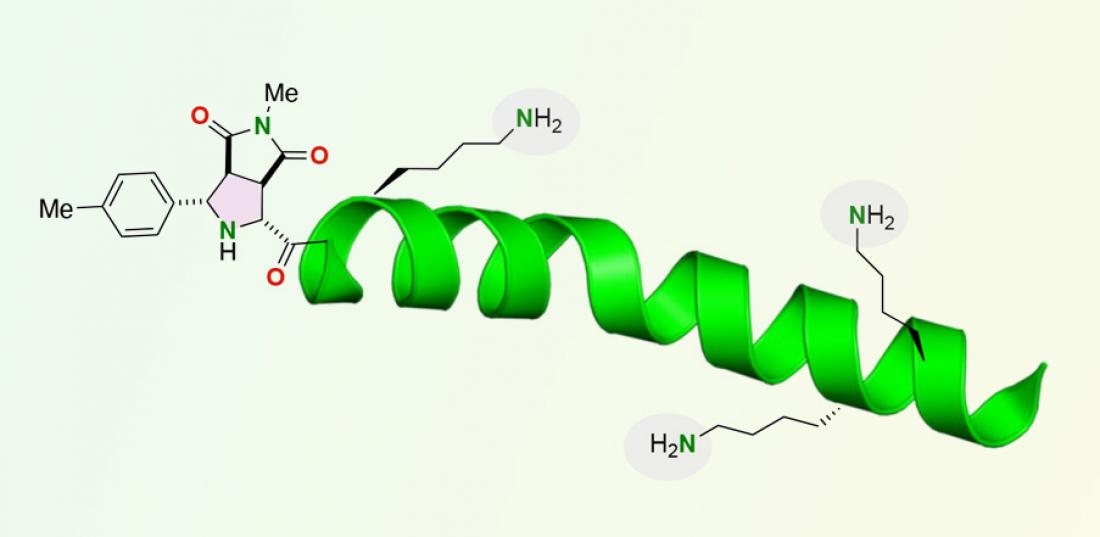
Peptides are short strands of amino acids that are increasingly used therapeutically, as biomaterials and as chemical and biological probes.
Peptides are short strands of amino acids that are increasingly used therapeutically, as biomaterials and as chemical and biological probes. The capacity to isolate, manipulate and label peptides and larger proteins is limited, however, by the ability to reliably attach functional molecules, such as fluorescent compounds, to peptides in locations that won't affect the three-dimensional structure and function of the short amino acid strand.
Researchers are most interested in adding functional molecules to the N-terminus, or the end of a peptide with a free amine group (NH2), of an amino acid strand in order to minimize the interference of functional molecules with the structure and function of the bound peptide. Earlier methods of attaching functional molecules to the N-terminus of peptides were insufficient for several reasons: 1) the functional groups would release from the peptide in human physiological conditions, 2) only one functional group could be attached to a peptide at a time, 3) attachment of functional molecules to peptides was not uniform or 4) reactions simply weren't efficient.
To address this issue, researchers from Tohoku University and Chuo University developed a unique chemical reaction to attach two distinct functional molecules to the N-terminus of a peptide with a glycine amino acid at the N-terminus. The researchers published their study in the January 28, 2024 issue of the journal Angewandte Chemie International Edition.
"The challenge [in modifying peptide structures] lies in achieving site-selective modification, particularly in the presence of highly reactive lysine residues. Our approach is noteworthy for its ability to exclusively functionalize the N-terminus of peptides, irrespective of lysine residues, resulting in structurally uniform conjugates in high yields. Furthermore, the three-component protocol facilitates the simultaneous installation of two functional molecules into a peptide," said Kazuya Kanemoto, senior author of the paper and assistant professor at the Graduate School of Pharmaceutical Sciences at Tohoku University in Japan.
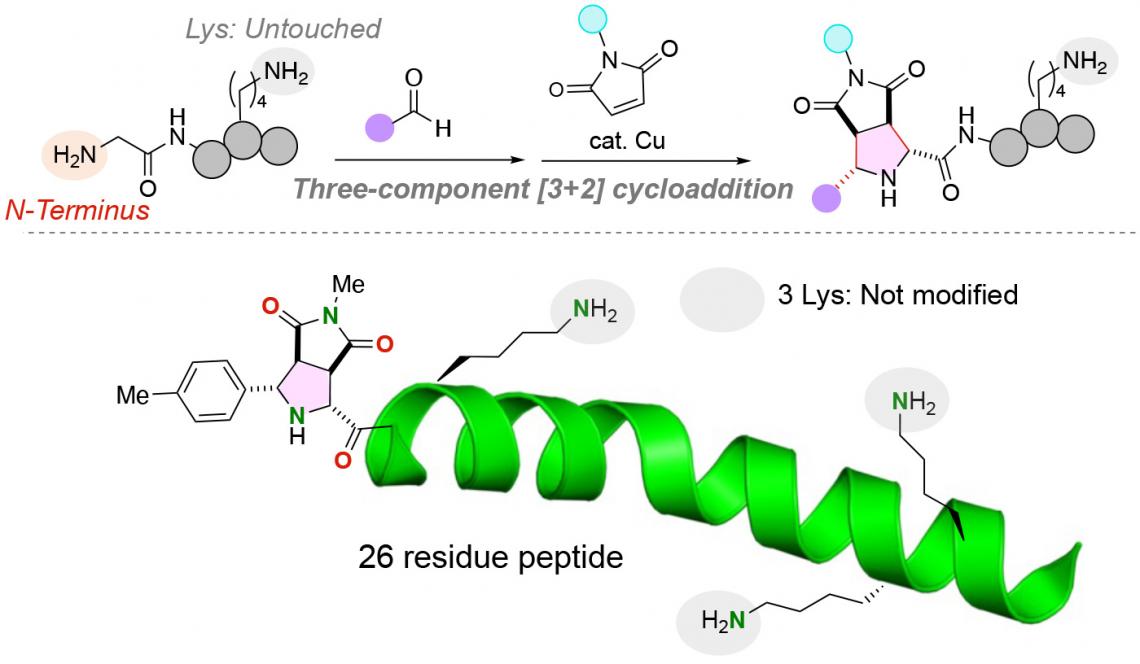
The team successfully attached the two distinct functional molecules to the glycine amino acid by using a copper catalyst in a three-component reaction of peptides, aldehydes (any organic compound with a carbon atom that shares a double bond with an oxygen atom, a single bond with a hydrogen atom and a single bond with another atom) and maleimides, molecules that are important building blocks in organic synthesis reactions. Remarkably, the reaction is performed in a single pot under mild conditions, resulting in a very efficient reaction with stable carbon-carbon bonds between the N-terminus of the peptide and the functional molecules.
An N-terminal specific three-component [3+2] cycloaddition proceeds without affecting the highly reactive lysine residues. This reaction has been successfully applied to polypeptides of up to 26 residues.
Lysine amino acids, in particular, have complicated the addition of functional molecules to the N-terminus of peptides. The functional group of lysine amino acids is an amine group that could potentially compete with the amine group present at the N-terminus of a peptide strand. Importantly, the chemical reaction developed by the research team labels only the N-terminus anime group of peptides even if a lysine amino acid, containing an alternative amine group, is present in the peptide.
The research team found that the N-terminal attachment of functional groups to peptides could be optimized for a variety of di-, tri- and oligopeptides, demonstrating the reaction's potential utility in labeling diverse peptides and potentially larger proteins for purification, detection and other purposes.
The researchers are already testing the function of peptides that have been modified through their new reaction to determine the suitability of the final product for various research and therapeutic purposes. "Our subsequent steps involve evaluating the biological activity of peptides prepared by this reaction. Additionally, we aim to extend the application of this site-selective dual modification protocol to larger peptides such as proteins and antibodies, showing promise for advancements in drug delivery," said Kanemoto.