Substrate mediated decomposition of Pd-NHC complexes under catalytic conditions.
Metal complexes that are stabilized by carbon-containing molecules, called N-heterocyclic carbene (NHC) ligands, have been successfully used in a number of catalytic reactions important for the synthesis of drugs, agrochemicals and materials for organic electronics.
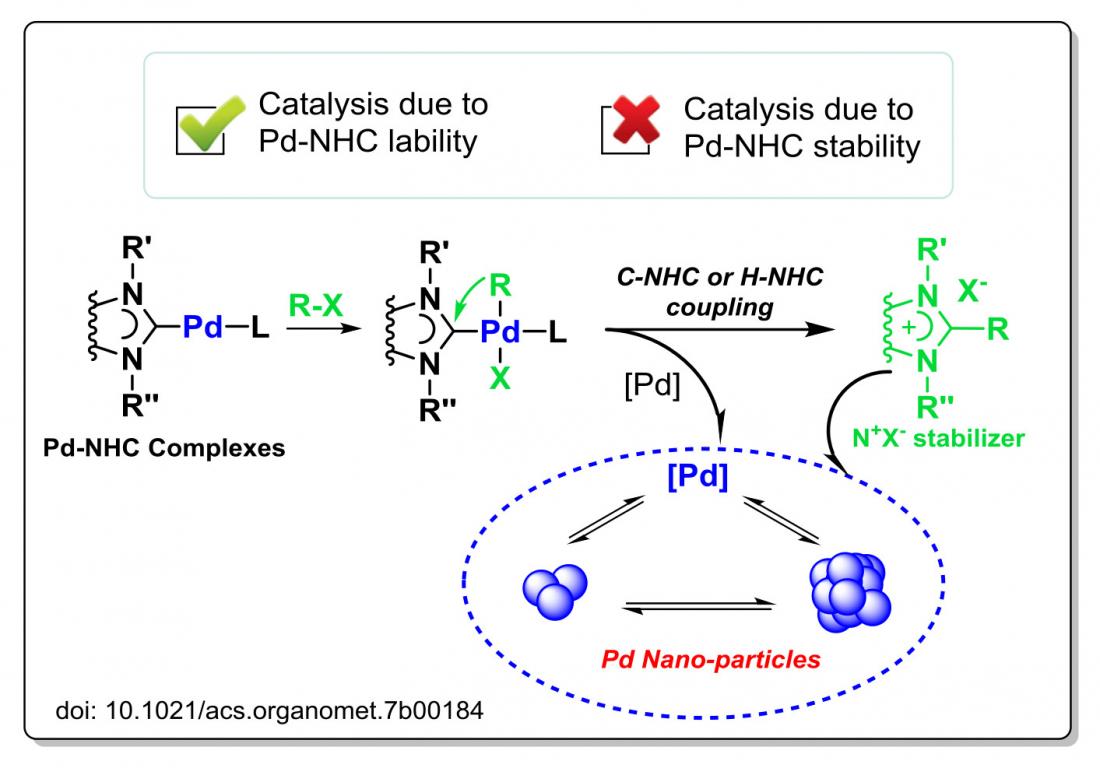
Researchers have long assumed that NHC ligands are strongly attached to metal centers not only in the initial complexes, but also under under catalytic conditions.
However, a recent study by Russian researchers has shown that complexes involving the metal palladium readily release their NHC ligands into solution during catalytic reactions, generating palladium clusters and nanoparticles.
The researchers studied the so-called catalytic Heck reaction, involving the addition of a compound, called an organic halide, to palladium-NHC complexes. The organic halide contains a halogen atom (X) bonded to a carbon atom that is part of an aryl group (R). When added to the palladium-NHC complexes, they turned to R-Pd(NHC)-X species.
They were surprised to find that the aryl group R readily coupled with the NHC ligand, leading to the dissociation of the ligand into solution.
A series of tests confirmed that nanoparticles that formed shortly after the beginning of this catalytic process played a key role in the reaction.
In this catalytic process, Pd-NHC complexes were not the actual catalyst. Instead, they were a ‘precursor’: a compound that participates in a chemical reaction to form other compounds.
Quantum chemical calculations predicted that degradation of the Pd-NHC complexes via the R-NHC coupling should occur easily. This was confirmed experimentally.
The NHC ligands are easily released into solution in the form of azolium salts, which play an important role in the stabilization of metal nanoparticles in solution. This reveals a new mode of operation of palladium-NHC systems, where catalysis is governed by the lability of the Pd-NHC framework, rather than its previously assumed stability.
The mechanism of several catalytic reactions may need to be reinvestigated as a result of these findings to assess the impact of the lability of metal-NHC complexes on these reactions.
The article, "A New Mode of Operation of Pd-NHC Systems Studied in a Catalytic Mizoroki-Heck Reaction", was published in the journal Organometallics (American Chemical Society) and was highlighted as a video cover (https://youtu.be/phwsqEl2CPw).
For further information please contact:
Prof. V.P.Ananikov <[email protected]>