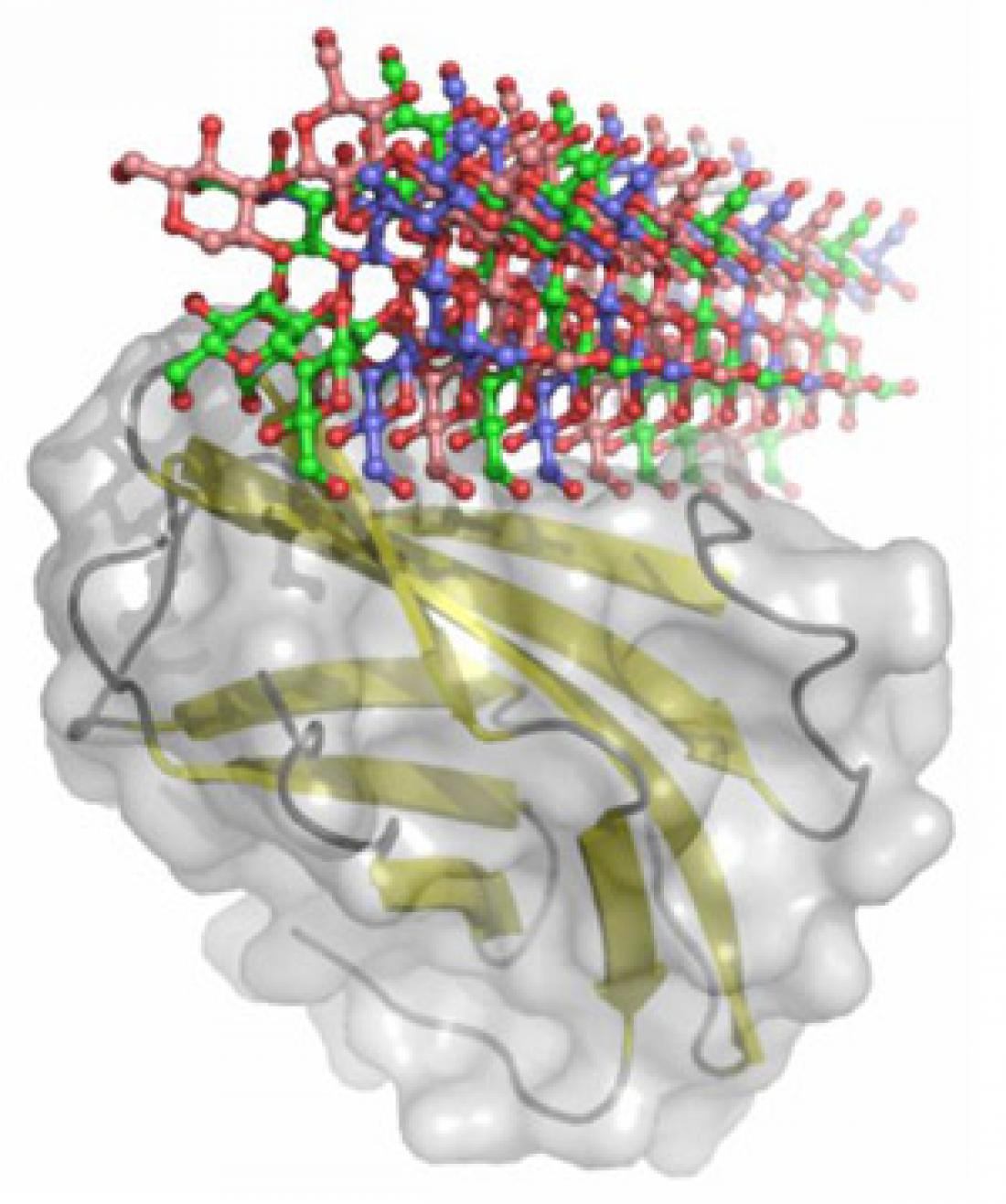
The β-glucan recognition protein (yellow) from Plodia interpunctella recognizes and binds to a triple helical β-glucan of invading pathogens.
Researchers in Japan have determined the structural basis of the molecular defense system that protects insects from pathogens, which provides clarity on the molecular binding that underpins this defense system.
Insects express pattern recognition receptors (PRRs) that provide an innate ability to detect fungi and plant pathogens. One of the PRRs, β-glucan recognition protein (βGRP), recognizes and binds to carbohydrate molecules called β-glucans that are synthesized by pathogens. Since little is known about how these molecules bind to each other, or about how the binding specificity is achieved, Yoshiki Yamaguchi of the RIKEN Advanced Science Institute and his colleagues used genetic engineering to produce the β-glucan-binding regions of βGRPs from two moth species, Bombix mori and Plodia interpunctella. They determined the structure of the receptors, both on their own and when bound to a β-glucan called laminarihexaose, using x-ray crystallography.
Analysis of the crystal structures revealed that the moth receptors recognize a complex of three laminarihexaoses bound to each other, and that their conformation barely changes when they are bound to laminarihexaoses. The analysis also revealed that the proteins from both species bind to laminarihexaoses in an identical way, via a characteristic structural motif, suggesting that the entire βGRP family shares a common binding mechanism.
Yamaguchi and colleagues also revealed that the laminarihexaose molecules attach to each other with hydrogen bonds that form an ordered and highly stable helical structure. Six precisely arranged monosaccharide (sugar) residues, spread across three chains, interact with the receptor binding site simultaneously, and are essential for the interaction.
To verify their findings, the researchers introduced point mutations at specific locations in the binding region of the Plodia interpunctella receptor. Four of the mutations abolished binding of β-glucan altogether, and four others weakened the binding interaction.
Typically, interactions between carbohydrates and proteins are relatively weak because they involve just two or three monosaccharide residues. The finding that the interaction between the receptors and β-glucan involves six residues explains why this interaction is so strong; it also explains the high specificity of the receptors.
Mammals do not produce β-glucans, but they circulate in the bloodstream of patients with diseases such as invasive aspergillosis, a rapidly progressive and often fatal fungal infection.
“Our findings will be used for the development of diagnosis and monitoring tools with high specificity toward a variety of β-glucans,” says Yamaguchi. “Detecting β-glucans in patients may be helpful for identifying infectious fungi, which could in turn be useful to tailor-make treatments for patients.”
The corresponding author for this highlight is based at the Structural Glycolobiology Team, RIKEN Advanced Science Institute
Reference:
Kanagawa, M., Satoh, T., Ikeda, A., Adachi, Y., Ohno, N. & Yamaguchi, Y. Structural insights into recognition of triple-helical β-glucan by insect fungal receptor. Journal of Biological Chemistry 286, 29158–29165 (2011).