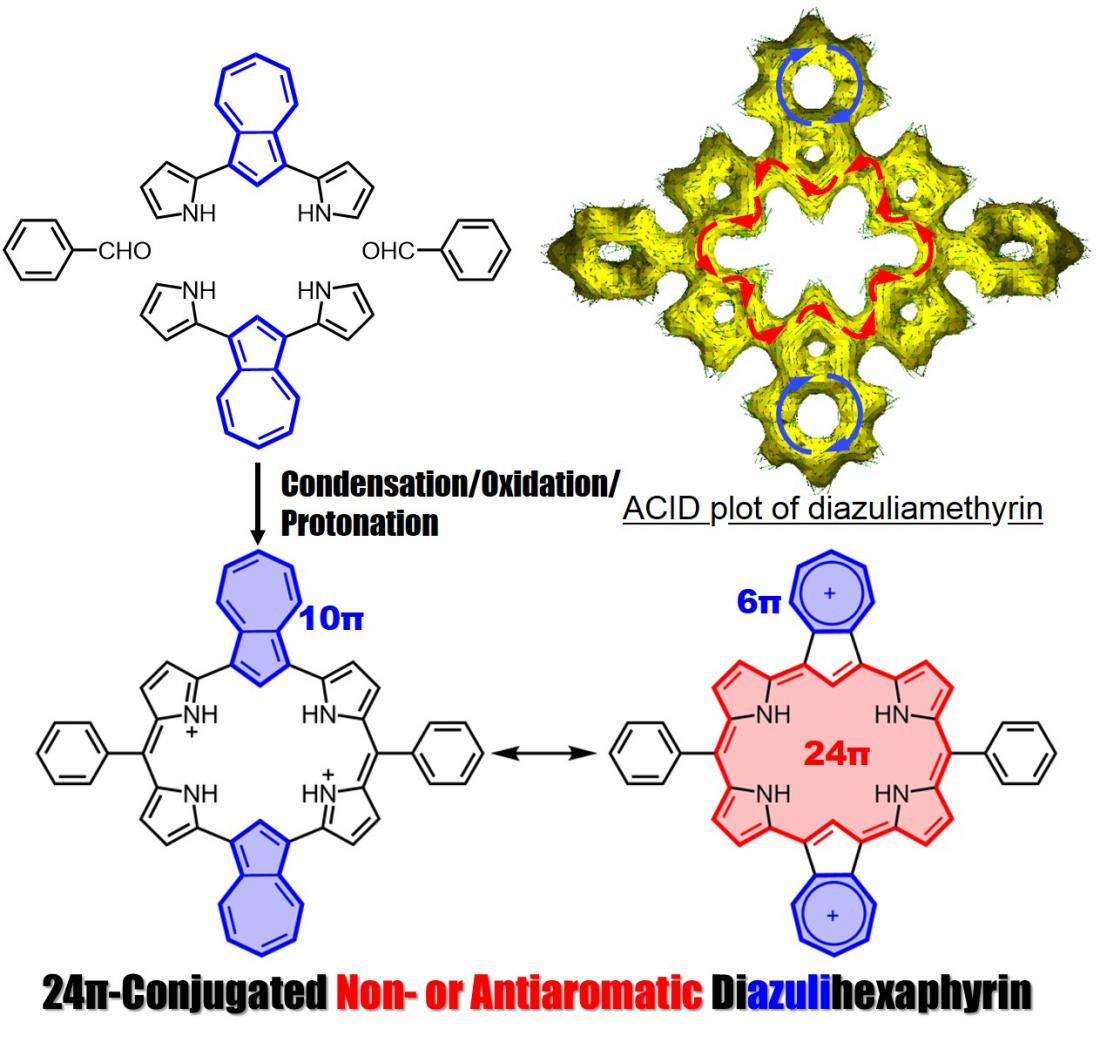
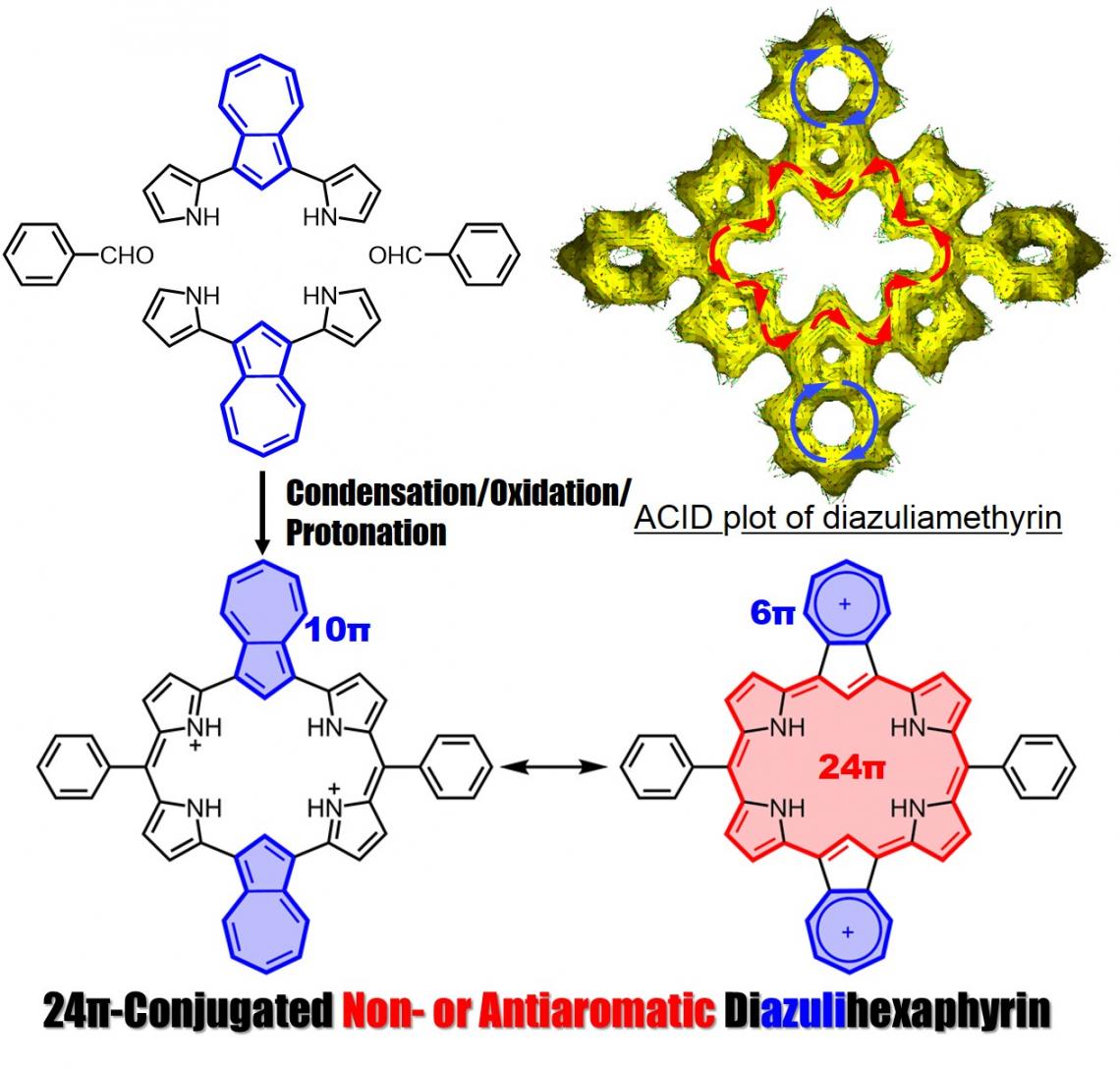
Synthesis of diazuliamethyrin
Profs. Okujima and Uno, in collaboration with Prof. Kobayashi at Shinshu University, reported their success in the synthesis of diazuliamethyrin, a core-modified hexaphyrin(1.0.0.1.0.0), and descibed its molecular structure, electronic structure and optical properties.
Amethyrin is a stable and antiaromatic ring-expanded porphyrin comprised of 6 pyrroles and 2 meso-bridged carbons. We successfully synthesized diazuliamethyrin via a “3+3” porphyrin synthesis. We confirmed it to have a 24pi non- or antiaromatic character by NMR, absorption, and MCD spectra analyses, and by TD-DFT calculations. Our findings were published on December 21, 2021 in Organic Letters.