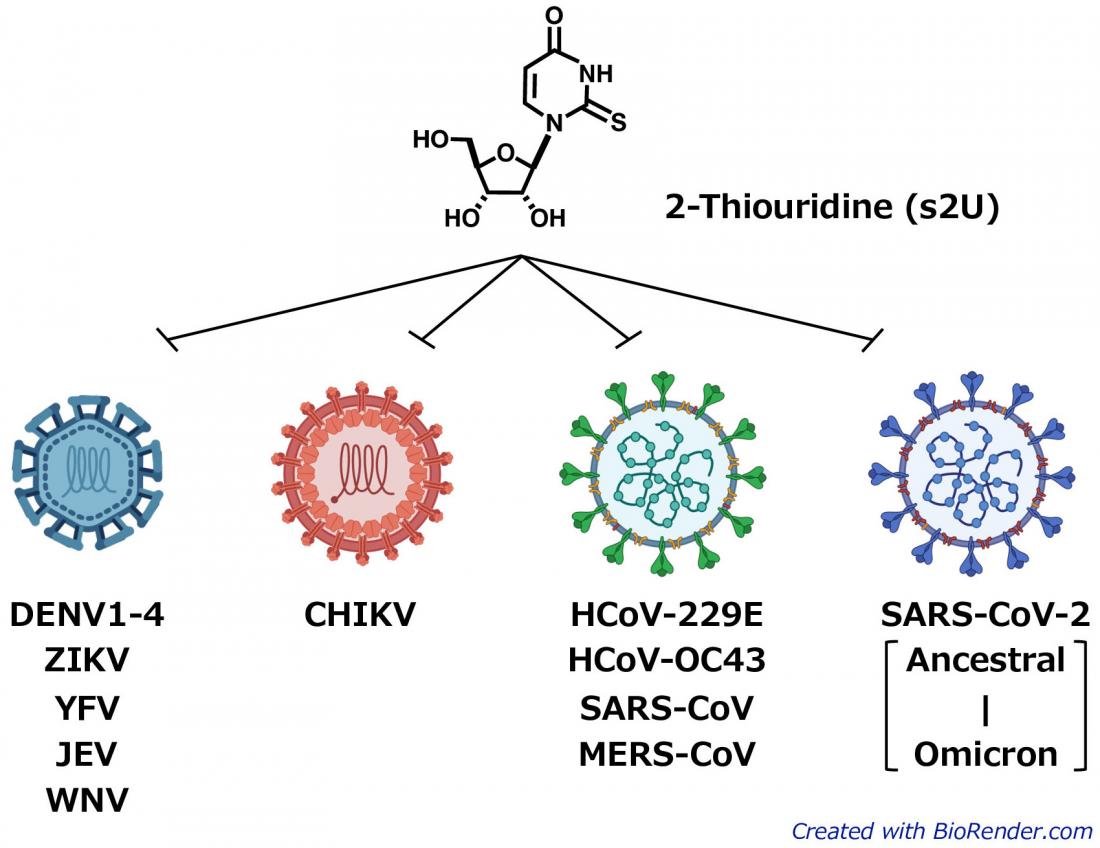
The newly identified 2-thiouridine (s2U) shows broad-spectrum antiviral activity against various ssRNA+ viruses including DENV, CHIKV, and SARS-CoV-2. (Kentaro Uemura, created with Biorender.com)
Positive-strand RNA viruses (ssRNA+ viruses) are a group of viruses which includes many disease-causing viruses such as dengue virus, Zika virus, yellow fever virus, Japanese encephalitis virus, West Nile virus, Chikungunya virus, SARS-CoV-2, and the rhinoviruses and coronaviruses that cause the common cold. Currently, no effective antiviral drugs targeting dengue virus have been approved for clinical use.
Researchers at Hokkaido University, led by Professor Katsumi Maenaka, Emeritus Professor Akira Matsuda, and Visiting Professor Akihiko Sato have identified 2-thiouridine (s2U), a uridine analogue, as a candidate for an ssRNA+ virus-targeting antiviral drug. Their findings, which also characterize its mode of action, were published in the journal Proceedings of the National Academy of Sciences.
“The genetic material of ssRNA+ viruses is positive-strand RNA,” explains Maenaka. “This means that it can function both as a genome, to replicate, and as messenger RNA, to synthesize proteins. Thus, upon infection, the most important protein synthesized is a viral RNA-dependent RNA polymerase (RdRp); this protein is an ideal drug target, as it is viral in origin and is not synthesized by healthy cells.”
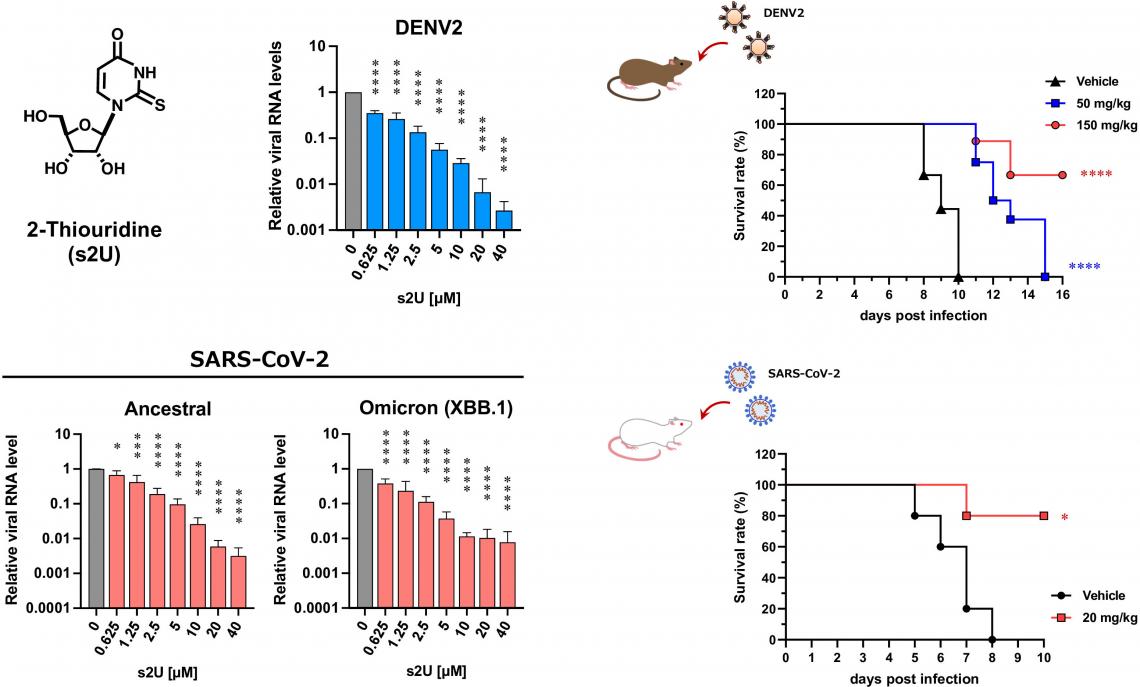
The researchers screened a library of 753 nucleoside analogs for antiviral effects. s2U was identified as strongly inhibiting viral replication—in cell cultures—in 10 ssRNA+ viruses, notably including the SARS-CoV-2 Delta and Omicron variants. Drug-resistant virus isolation tests showed that s2U acted on a specific amino acid in the viral RdRp, inhibiting the synthesis of viral RNA. s2U did not have any toxic effects on the cells at therapeutic concentrations.
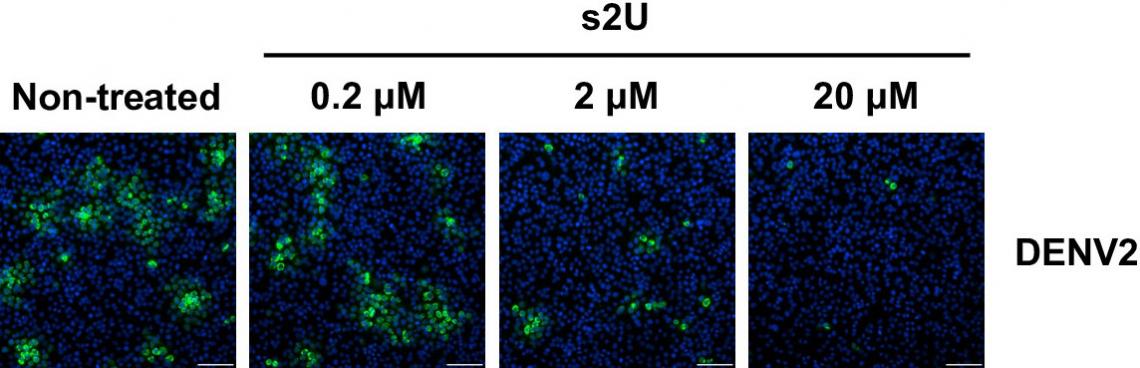
Dose-response inhibition of viral protein expression (green) in the DENV2-infected cells. (Kentaro Uemura et al., PNAS, October 13, 2023)
The researchers also tested the efficacy of s2U in mice models for dengue and COVID-19. In both models, s2U significantly inhibited viral replication and increased survival rates. “In mice, one intravenous injection per day, or two oral administrations per day, maintained a therapeutically effective concentration of s2U in blood plasma,” Matsuda elaborated. “In addition, s2U did not have any toxic or mutagenic effects.”
Dose-response inhibition of viral replication and in vivo evaluation for DENV (top) and SARS-CoV-2 (bottom). (Kentaro Uemura et al., PNAS, October 13, 2023)
This study demonstrated that s2U exhibits strong, safe, broad-spectrum antiviral activity against ssRNA+ viruses by inhibiting viral RdRp activity. “Since it is effective against the ancestral SARS-CoV-2 and the Delta and Omicron variants, we conclude that it will retain this effectiveness against future variants,” said Sato. s2U now needs to be subjected to the process of human trials, to confirm its effectiveness.