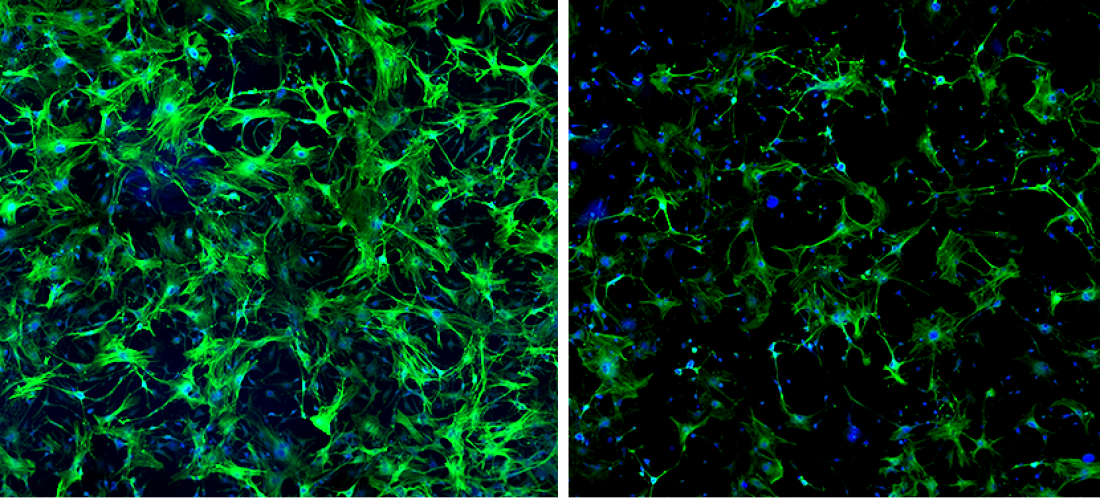
Macrophages lacking WWP2 greatly reduce the “triggering” of fibroblasts during the development of cardiac fibrosis. Microscopy images show the immunostaining for ACTA2, a protein marker for fibroblasts activation (in green). In this experiment, primary cardiac fibroblasts were “triggered” towards their fibrotic state using the factors secreted by either stimulated macrophages expressing WWP2 (left panel) or by stimulated macrophages lacking WWP2 (right panel). When triggered with macrophages lacking WWP2, cardiac fibroblasts exhibited reduced activation towards their fibrotic state, which is typically associated with uncontrolled scarring of the heart tissue.
SINGAPORE, 9 December 2022 – Scientists at Duke-NUS Medical School have identified a gene that controls the behaviour of a specific type of cardiac macrophage responsible for excessive scarring during the early phases of common heart diseases or cardiomyopathies. When the gene, called WWP2, is blocked, heart function is improved and scar tissue formation is slowed, delaying the progression to heart failure.
“Scarring or fibrosis of the heart, as in non-ischaemic cardiomyopathies, is a progressive condition and global health concern,” explained Associate Professor Enrico Petretto, Director of Duke-NUS’ Centre for Computational Biology and a systems geneticist with the School’s Cardiovascular & Metabolic Disorders (CVMD) Programme. “In its earliest stages, it is characterised by an inflammatory phase, so intervening at that point could significantly delay disease progression.”
Assoc Prof Petretto and colleagues in Singapore, China and the UK had been studying the function of WWP2 in fibrotic diseases for several years, first discovering that it is a significant driver of scarring when it is expressed in fibroblasts—the cells that make scar tissue. In their latest findings, published in Nature Communications, his team turned their attention to the early stage of the disease.
Using single cell RNA sequencing, the team found when fibrosis is triggered, a wide range of different macrophages—immune cells that clear foreign material in the body—are activated in a preclinical model of heart disease. While macrophages are mostly known for their role in removing cancer cells, microbes and cellular debris, they also help with the regeneration of healthy muscle cells. However, a subset of these cardiac macrophages are controlled by WWP2. These WWP2-expressing macrophages actively promote scarring by triggering local cardiac cells (fibroblasts) to produce collagen in an uncontrolled manner, fuelling scar tissue formation.
“In this latest study, we focused on the ‘cross-talk’ that happens between macrophages and fibroblasts in the early stages of fibrogenesis,” said Dr Chen Huimei, Senior Research Fellow with the CVMD Programme, who is first and co-corresponding author of the paper. “We found that when WWP2 is expressed in macrophages, these cells ‘irritate’ fibroblasts which leads to uncontrolled scarring.”
When macrophages did not express WWP2, on the other hand, the team observed reduced infiltration of pro-fibrotic macrophages into the heart, and the action of repair macrophages was better sustained with clear beneficial effects on cardiac tissue and function during the later stages of the disease.
“Targeting WWP2 is like throwing a blanket over the fire—it removes the oxygen from the flames before the whole house burns down,” explained systems biologist Associate Professor Jacques Behmoaras, from the CVMD Programme, a co-corresponding author of the study who is also Reader in Immunogenetics with the Department of Immunology and Inflammation, Imperial College London. “Blocking WWP2’s function in this subset of cardiac macrophages is enough to slow—or even stop—the scarring.”
The team is developing a small molecule inhibitor against WWP2 that can achieve just that. Rather than depleting all macrophages indiscriminately, which has shown deleterious effects, the Duke-NUS team is targeting WWP2, which works specifically on those pro-fibrotic macrophages and activated fibroblasts to halt scarring of the damaged heart.
“Because WWP2 plays a double role in the formation of scar tissue, blocking it ‘kills two birds with one stone’, by dampening inflammation and scarring at once. And with its additional advantage of boosting the beneficial tissue repair macrophages, WWP2 becomes a very attractive therapeutic target,” added Assoc Prof Petretto, the senior author of the study. “We are now developing small molecule inhibitors that target a specific form of the WWP2 protein, which have already shown promising anti-fibrotic results in cells. We believe these could hold therapeutic potential for treating fibrotic conditions like non-ischaemic cardiomyopathies, and may prove effective in other fibrotic diseases where WWP2 is involved.”
References:
- Chen, H., Chew, G., Devapragash, N. et al. The E3 ubiquitin ligase WWP2 regulates pro-fibrogenic monocyte infiltration and activity in heart fibrosis. Nat Commun 13, 7375 (2022). https://doi.org/10.1038/s41467-022-34971-6
- Chen, H., Moreno-Moral, A., Pesce, F. et al. WWP2 regulates pathological cardiac fibrosis by modulating SMAD2 signaling. Nat Commun 10, 3616 (2019). https://doi.org/10.1038/s41467-019-11551-9