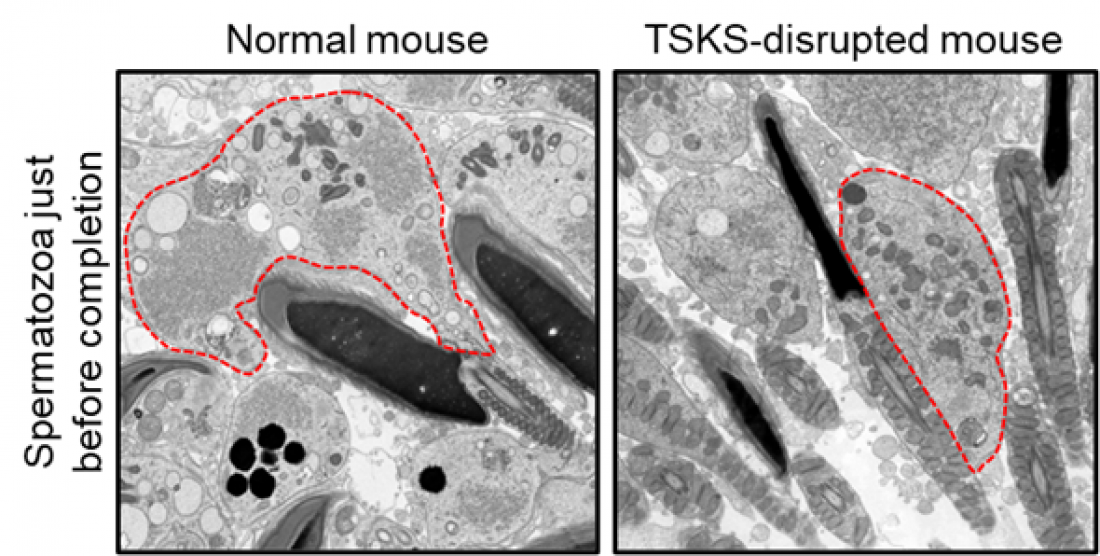
Fig. 1
Electron microscopic image of a sperm just before completion in the testis. In normal mice, the cytoplasm indicated by the red dashed line is eliminated just before sperm completion. In TSKS-disrupted mice, however, the cytoplasm is not eliminated and sperm with an abundance of cytoplasm are produced, resulting in male infertility.
Researchers from Osaka University identify the role of a protein in the elimination of sperm cytoplasm and reveal its effects on the fertility of males
Osaka, Japan – In order to achieve good times in their races, many Olympic swimmers wear swimsuits that are low in water resistance. Similarly, spermatozoa, the male reproductive cells, possess a “streamlined” structure to travel more smoothly through the female reproductive tract. Now, researchers in Japan have shed new light on a key protein involved in this process.
In a new study published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS), researchers from Osaka University have identified the role of a protein called testis-specific serine kinase substrate (TSKS) in the process of spermiation, or the release of mature spermatozoa.
During reproduction, spermatozoa must travel through the female reproductive tract to reach the oocyte, or egg, in order for fertilization to occur. To better facilitate this process, spermatozoa have a “streamlined morphology” that is achieved by eliminating sperm cytoplasm. While this process has been observed in previous studies, the molecular mechanisms underlying it are not fully understood. This led the research team from Osaka University to explore a mouse model targeting TSKS, which is localized to membraneless structures called nuage.
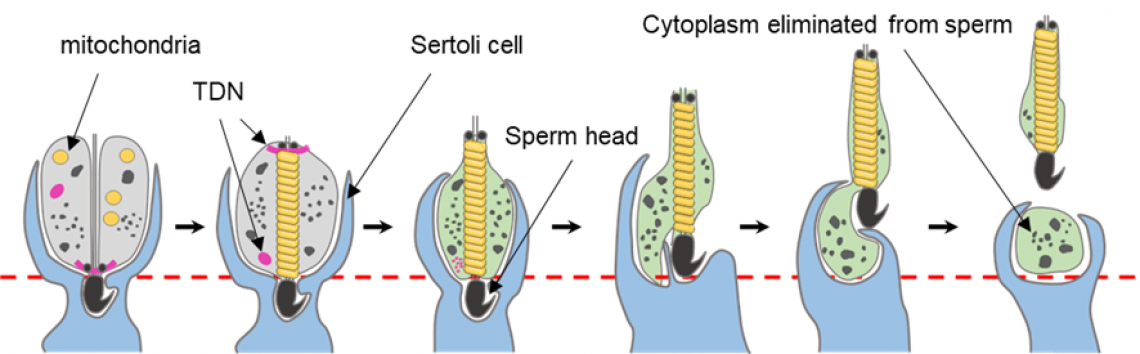
Fig. 2
Process of cytoplasmic elimination in sperm.
Sperm is formed in the testis. During the formation of sperm, Sertoli cells surround the sperm. The eliminated cytoplasm is left in the Sertoli cells.
“Using genome editing technology, we developed a mouse model in which TSKS has been disrupted,” says co-lead author of the study Keisuke Shimada. “We found that spermatozoa from the mice with disrupted TSKS failed to develop a streamlined form, resulting in male infertility.”
The researchers analyzed the spermatozoa from TSKS knockout mice and found that these sperm were unable to produce two specific types of nuage called reticulated body (RB) and chromatid body remnant (CR). Without these nuage, the TSKS-disrupted sperm could not properly eliminate their cytoplasm. Additionally, the researchers observed that the presence of excess residual cytoplasm led to apoptosis, or cell death, in these spermatozoa.
“Our results showed that generation of RB and CR nuage is dependent on TSKS,” says co-lead author Soojin Park. “TSKS is required for sperm to eliminate cytoplasm and adapt a streamlined, tadpole shape. This applies to humans as well, as TSKS is also present in human spermatozoa.”
This discovery of the role of TSKS on the formation of streamlined spermatozoa offers more clues on one mechanism behind male infertility. The findings from this study may translate to the development of diagnostic tests and male contraceptives.
###
The article, “TSKS localizes to nuage in spermatids and regulates cytoplasmic elimination during spermiation,” was published in PNAS at DOI: https://doi.org/10.1073/pnas.2221762120
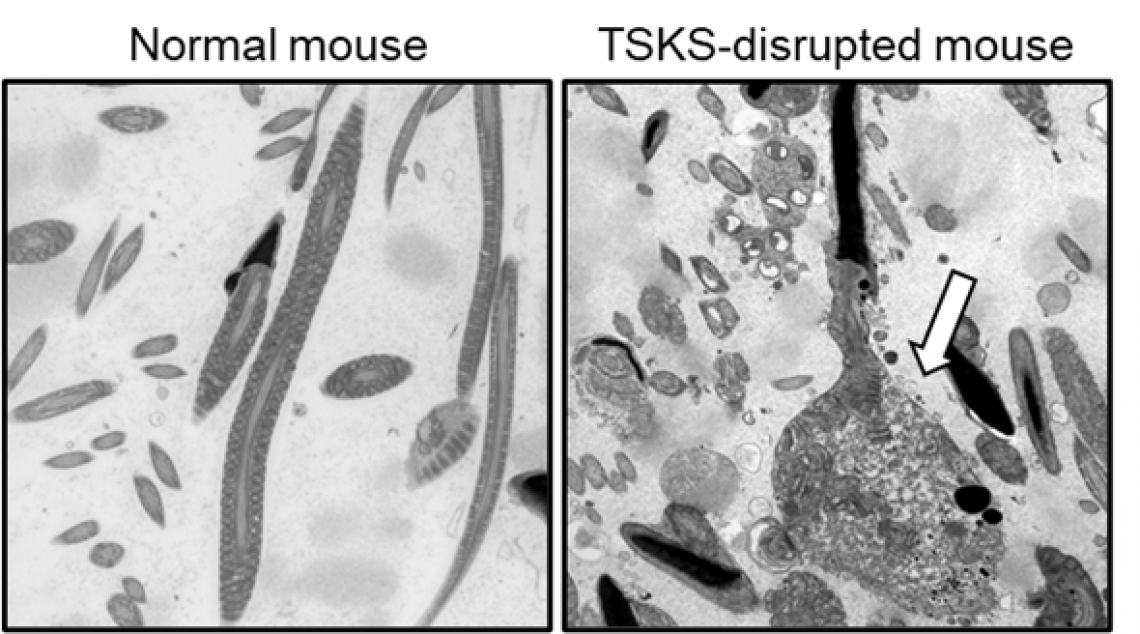
Fig. 3
Electron microscopic image of sperm in the epididymis.
Sperm from TSKS-disrupted mice show an apoptotic reaction and degradation in the cytoplasm (arrows).
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en