The molecules synthesized in this study form different isomers when irradiated with blue light. (Photo: Akira Katsuyama)
Researchers at Hokkaido University, led by Assistant Professor Akira Katsuyama and Professor Satoshi Ichikawa at the Faculty of Pharmaceutical Sciences, have extended the toolkit of synthetic chemistry by making a new category of molecules that can be induced to undergo an internal rotation on interaction with light. Similar processes are believed to be important in some natural biological systems. Synthetic versions might be exploited to perform photochemical switching functions in molecular computing and sensing technologies, or in bioactive molecules including drugs. They report their findings in Nature Chemistry.
“Achieving a system like ours has been a significant challenge in photochemistry,” says Katsuyama. “The work makes an important contribution to an emerging field in molecular manipulation.”
Insights into the possibilities for light to significantly alter molecular conformations have come from examining some natural proteins. These include the rhodopsin molecules in the retina of the eye, which play a crucial role in converting light into the electrical signals that create our sense of vision in the brain. Details are emerging of how the absorption of light energy can induce a twisting rearrangement of part of the rhodopsin molecule, required for it to perform its biological function.
“Mimicking this in synthetic systems might create molecular-level switches with a variety of potential applications,” Katsuyama explains.
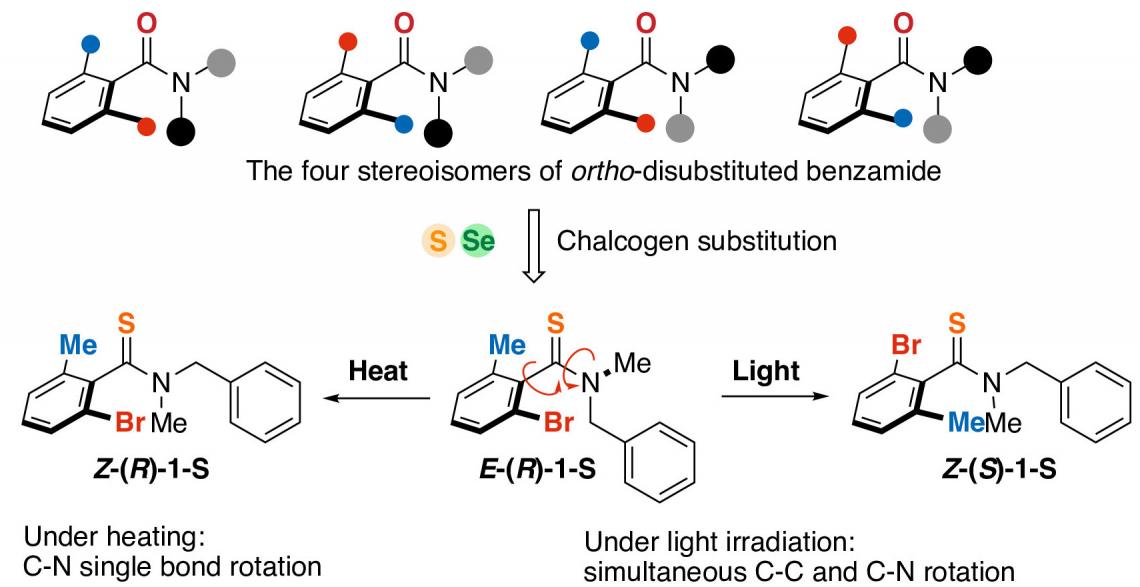
A key innovation by the Hokkaido team was to achieve photo-induced (i.e., light-driven) rotation of molecular groups around a series of chemical bonds that incorporate a nitrogen atom together with other bonded carbon atoms.
The rotational properties were enabled by adding molecular components that contained an atom from the ‘chalcogen’ group of elements in the periodic table, specifically sulfur or selenium, to a simple organic molecule: an amide compound. This brought a new level of control and versatility to synthetic photo-induced rotational systems.
In this study, sulfur or selenium atoms were introduced into a benzamide derivative (top). The resulting molecules underwent isomerization when exposed to light or heat. (Shotaro Nagami, et al. Nature Chemistry. February 28, 2024)
Some of the chemical groups that rotate around the central bonds were relatively large, based on rings of six bonded carbon atoms. This facilitated the large-scale molecular changes that might be required for practical use in molecular switching systems.
In addition to demonstrating the photo-induced changes, the team also performed theoretical calculations that gave insights into the likely mechanisms by which the rearrangements proceeded. The team also explored the effects of temperature on the transformations. The combination of theoretical and experimental work should help guide future research towards exploring and controlling modifications to the systems already achieved.
"Our next research priority is focused on the potential of our methods for making new bioactive molecules activated by light. These could be applied in biological research or possibly developed as drugs,” Ichikawa concludes.
Using light to activate the conformational changes allows control over where and when the changes occur. This could be vital for precisely targeted applications in biological systems, including eventual therapeutic possibilities.