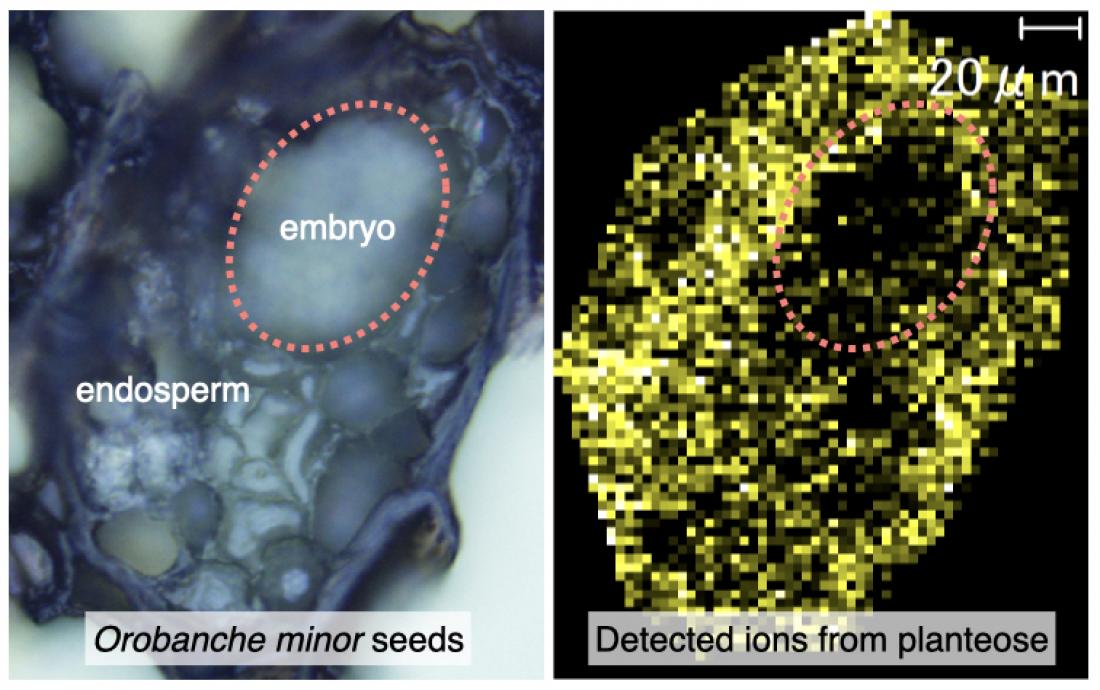
Figure 1. Mass Imaging. Visualization of planteose in dried Orobanche minor seeds. Bright field seed section image (left); Mass image from a precursor ion m /z 526.17 (right).
Osaka, Japan—Witchweed (Striga spp.) and broomrapes (Orobanche and Phelipanche spp.) are root parasitic weeds that inflict major losses in agriculture globally. Being obligate parasitic flowering plants in nature, they parasitize other autotrophic plants of agricultural importance. The plants are attached to their host by means of haustoria, which transfer nutrients from the host to the parasite. Weeds reduce crop yield by competing for resources (nutrients, water and photosynthetically active radiation), by allelopathic effects, and they reduce food, feed, and fiber quality.
According to a review by Chris Parker; based on rigorous sampling, there are no reliable global figures for the total area affected by Orobanche or Striga. However, an estimated 16 million hectares were ‘at risk’ of Orobanche attack in the Mediterranean and West Asia region. The corresponding figure for Striga in Africa was 44 million hectares, while the total loss of revenue from maize, pearl millet and sorghum are almost USD 2.9 billion. More recent figures suggest that 50 million hectares and 300 million farmers are affected by Striga species in Africa, with losses amounting to USD 7 billion.
It is difficult to control parasitic weeds because they are obligate parasites. One potential method of control is the use of growth modulators which are specific to root parasitic weeds. Understanding the physiological mechanisms that occur during the life cycles of root parasitic weeds is crucial for identifying specific growth modulator targets.
Planteose (a storage carbohydrate) metabolism is a possible target for root parasitic weed control. In a previous research Associate Professor Atsushi Okazawa and his collaborators revealed that planteose metabolism was activated after perception of strigolactones (a class of plant hormones that stimulate branching in plants and the growth of symbiotic arbuscular mycorrhizal fungi) in germinating seeds of Orobanche minor. Nojirimycin (a potent inhibitor of α-glycosidase) inhibited planteose metabolism and impeded seed germination of Orobanche minor, indicating that planteose metabolism is a possible target for root parasitic weed control.
In a more recent study, this team of scientists based at Osaka Prefecture University, investigated α-galactosidases (AGALs) activities during seed germination of Orobanche minor. They also studied planteose distribution in the dry seeds using matrix-assisted laser desorption/ionization–mass spectrometry imaging.
Planteose was found in tissues surrounding the embryo but not within it, indicating that it may have a role as a storage carbohydrate. Biochemical experiments and molecular characterization of a α-galactosidase family member, OmAGAL2, indicated the enzyme is involved in planteose hydrolysis in the apoplast around the embryo after the perception of strigolactones to provide the embryo with essential hexoses for germination. These results indicated that OmAGAL2 is a potential molecular target for root parasitic weed control.
Mass spectrometry images obtained for two fragment ions were almost identical, indicating that these fragment ions were all generated from a single source, planteose. The authors also provided visual aids demonstrating that planteose is distributed in the endosperm, perisperm and seed coat in the dry seeds of Orobanche minor (Figure 1), which coincides with its role as a storage carbohydrate.
In summary, the discovery of this study elucidates that (i) planteose is distributed in Orobanche minor dry seeds and its physiological role is elusive, (ii) during seed germination of root parasitic weeds, planteose is rapidly hydrolyzed after perception of strigolactones (SLs), which is indicative of its role as a storage carbohydrate (iii) tissues surrounding the embryo, namely the endosperm, perisperm, and seed coat, play roles in nutrient supply in root parasitic weeds.
Moreover, the novelty of this study lies in the fact that; for the first time, the authors visualized the distribution of the storage carbohydrate (planteose) in seeds of a root parasitic weed.
The article “Involvement of α-galactosidase OmAGAL2 in planteose hydrolysis during seed germination of Orobanche minor” was published on 01 December 2021 in Journal of Experimental Botany.
https://doi.org/10.1093/jxb/erab527
In addition, these findings also build up on the following previously published articles:
“The effect of nojirimycin on the transcriptome of germinating Orobanche minor seeds”
https://doi.org/10.1584/jpestics.D20-057
“Planteose as a storage carbohydrate required for early stage of germination of Orobanche minor and its metabolism as a possible target for selective control”
https://doi.org/10.1093/jxb/erv116
“Observations on the current status of Orobanche and Striga problems worldwide”
https://doi.org/10.1002/ps.1713
###
About Osaka Prefecture University, Japan
Osaka Prefecture University (OPU) is one of the largest public universities in Japan.
OPU comprises three campuses, with a main campus in Sakai, Osaka. With four colleges for undergraduate students and seven graduate schools, the university offers stellar education in a myriad of fields like engineering, life and environmental sciences, science, economics, humanities and social sciences, and nursing. Not just this, the university also houses various international students, who can enrich their lives with opportunities for internships and exchange programs.
In April 2022, OPU will unite with Osaka City University (OCU) to form Japan’s largest public university, Osaka Metropolitan University.
For more details, please visit:
Osaka Prefecture University (OPU): https://www.osakafu-u.ac.jp/en
Osaka Metropolitan University (OMU): https://www.upc-osaka.ac.jp/new-univ/en-research/