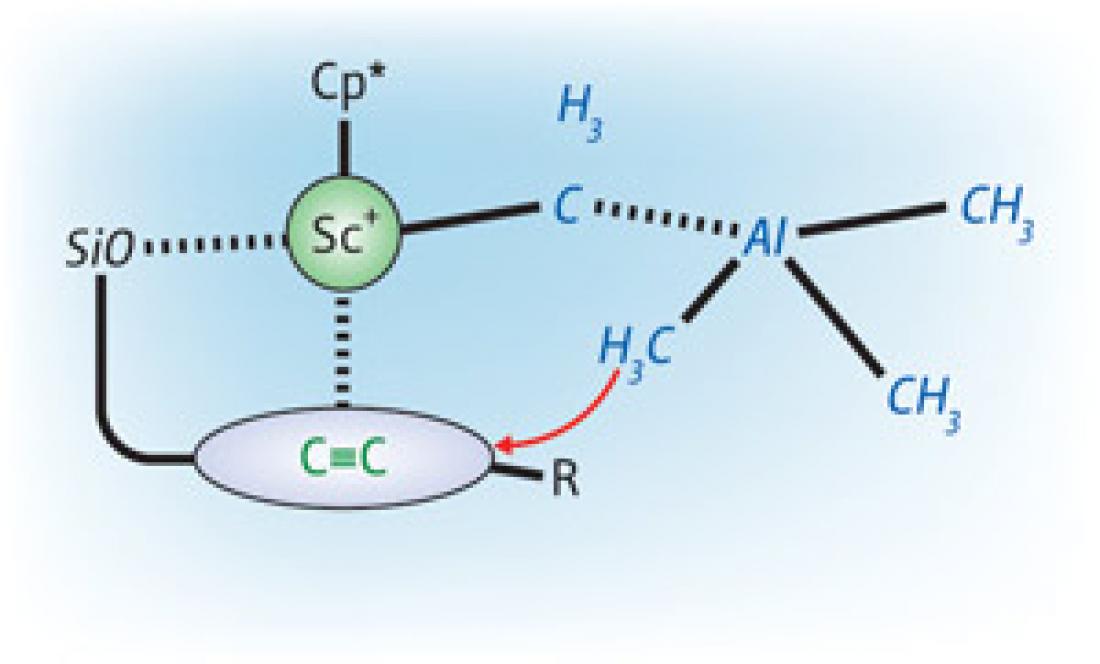
Figure 1: The interaction between a scandium-based catalyst (Sc+–Cp*) and a silyl ether group (SiO) allows the highly selective addition of methylaluminum (blue structure) to carbon–carbon triple bonds.
Subtle electronic differences between metals in the periodic table can lead to radical changes in chemical reactivity. Now, a research team led by Zhaomin Hou from the RIKEN Advanced Science Institute, Wako, has found that scandium, a seldom-studied rare-earth metal, enables the catalytic addition of functional groups to unsaturated carbon bonds with better selectivity than other metals1—a boon to chemists seeking precise control over molecular assembly.
Hou and his team are experts in the field of rare-earth materials, and recently discovered that a so-called ‘half-sandwich’ complex, comprising a scandium cation and a pentagonal carbon ring, could efficiently catalyze production of long polymer chains.
“Our scandium complex acted as an excellent catalyst for olefin polymerization, with unprecedented activity and selectivity,” says Hou. Because the scandium complex targeted unsaturated carbon bonds during the polymerization process, the researchers realized its enormous potential in other important synthetic reactions, such as carbometalation.
During carbometalation, a metal catalyst helps an organic unit, such as a methyl group, and a metal—commonly aluminum—to add to a carbon–carbon double or triple bond. Researchers can then replace the metal with another molecular group, making carbometalation an effective way to construct carbon-based frameworks containing multiple, branched functional units.
What is difficult, though, is controlling the regioselectivity of the catalytic addition—the precise positions where the organic and metal units add to the unsaturated carbon bonds. When the team first attempted carbometalation with the scandium catalyst and a typical triple-bonded carbon molecule, it achieved only moderate regioselectivity, similar to other transition metal catalysts.
However, when the researchers tethered a silyl ether—a group containing silicon, oxygen, and hydrocarbon atoms—to the end of the triple-bonded carbon substrate, the carbometalation proceeded with extremely high regioselectivity; over 99% of the final product was isolated as a single chemical isomer. Further experiments revealed that the combination of a silyl ether tether group and a scandium-based catalyst enabled controllable carbometalation on numerous unsaturated organic molecules—in many cases, with higher regioselectivity than any other catalyst.
According to Hou, the unprecedented selectivity achievable through this method is due to a balanced interaction between the oxygen atom of the silyl ether adduct and the scandium cation. “This interaction should not be too strong,” he says, “otherwise coordination and insertion processes around unsaturated carbon–carbon triple and double bonds would be hampered.”
The researchers are currently exploring new ways to utilize rare-earth complexes for chemical transformations involving carbon and other elemental bonds.
The corresponding author for this highlight is based at the Organometallic Chemistry Laboratory, RIKEN Advanced Science Institute