The findings represent a complete paradigm shift in our understanding and approach towards macrophage mediated diseases, such as neurodegenerative diseases in particular.
In this collaborative study co-led by A*STAR’s Senior Principal Investigator, Dr Florent Ginhoux, as well as Prof Lan Yu and Prof Bing Liu from Jinan University, the team characterized the earliest macrophages in humans, found that they come from a non-hematopoietic stem cell lineage, and that the earliest microglia in humans come from these cells. These findings represent a complete paradigm shift in our understanding and approach towards macrophage mediated diseases, such as neurodegenerative diseases in particular, and bridges observations in animal models with human biology.
a) Macrophages in the early human embryo already cluster together according to their distinct tissue resident identities. b) Looking specifically at cells before the emergence of hematopoietic stem cells that will eventually seed the bone marrow, we found that macrophages are already present, with 2 main lineages that contribute to them. CS = Carnegie Stage, a system used to measure human embryonic development. CS11 corresponds to roughly 24 days post-conception, while CS17 corresponds to roughly 38 days post-conception.
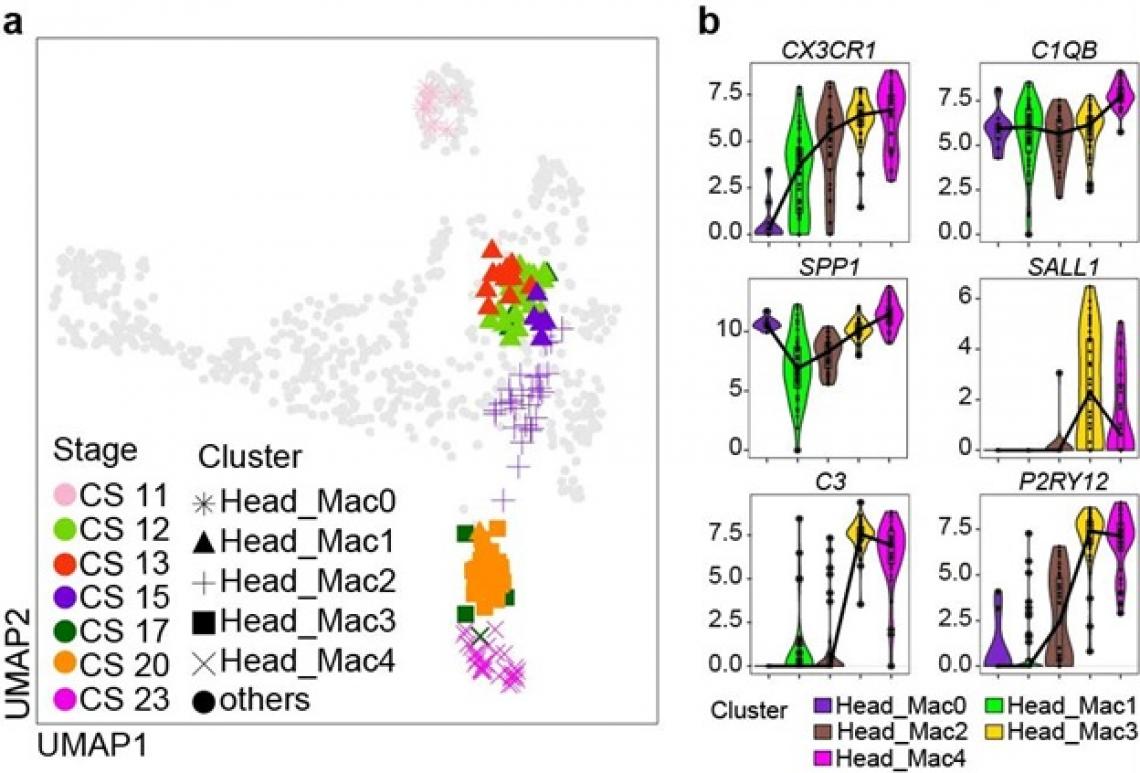
a) The earliest macrophages found in the human embryonic head shares similar features as those found in the yolk sac. We were able to trace their development from macrophages (at CS11) into microglia (at CS23). b) These human embryonic microglia expresses genes such as CX3CR1, SALL1 and P2RY12 in similar pattern to what we previously observed in mouse embryonic microglia. CS = Carnegie Stage, a system used to measure human embryonic development. CS11 corresponds to roughly 24 days post-conception, while CS23 corresponds to roughly 56 days post-conception.
a and b) We found that in addition to monocyte/macrophages, these progenitors could also give rise to neutrophils. Using monocle, we predicted their differentiation trajectories from progenitors into both monocytes and neutrophils. c) We were also able to map their specific gene expression changes across this process.
For more details about this study that was published in Nature, please visit:
https://doi.org/10.1038/s41586-020-2316-7
From top left: Professor Lan Yu, Jinan University/Shi Hui, Academy of Military Medical Science/Bian Zhilei, Jinan University/Huang Tao, Academy of Military Medical Science/Gong Yandong, Academy of Military Medical Science
From bottom left: Adjunct Professor Liu Bing, Jinan University/Dr Florent Ginhoux, Singapore Immunology Network/Christopher Lee, Singapore Immunology Network