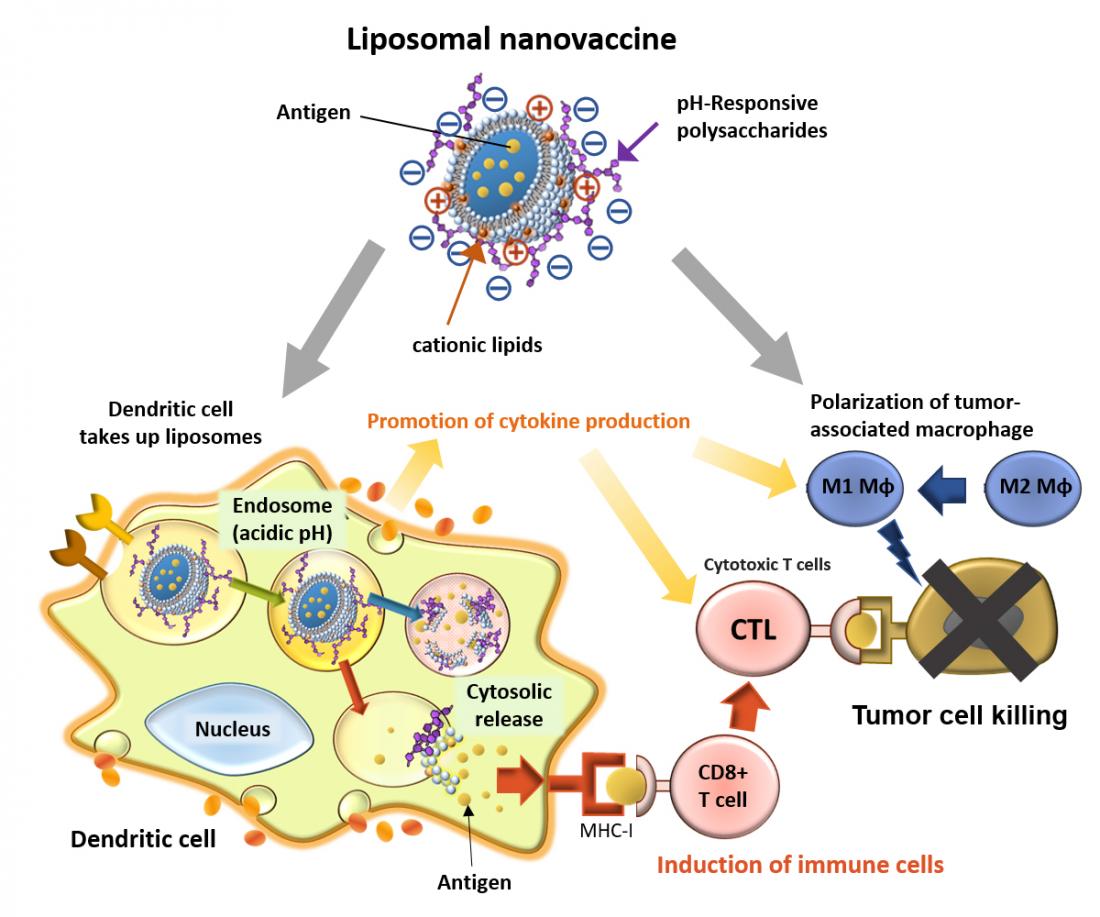
Cationic lipid-introduced pH-responsive polysaccharide-modified liposomes to achieve efficient antigen delivery and highly activation of antigen presenting cells towards induction of antigen-specific immune responses.
Cancer immunotherapies, such as immune checkpoint inhibition therapy, have been attracting attention in recent years as new methods for treating cancer. However, immune checkpoint inhibition therapy is only effective in 20%–30% of cancer patients, so developing better drug delivery systems to induce anticancer cellular immunity is necessary.
A research group led by Associate Professor Eiji Yuba of the Graduate School of Engineering at Osaka Metropolitan University has been developing an antigen carrier, using liposomes with pH-responsive polysaccharides on their surface to carry cancer antigens to dendritic cells. By incorporating positively charged cationic lipids into liposomes, the research group has now successfully developed their new drug delivery system, to strongly activate cellular immunity, using one-tenth of the amount of antigen that was required previously. The results were published by Elsevier in the Journal of Controlled Release, on Monday, October 31, 2022.
To increase the efficacy of these liposomal nanovaccines, it is necessary for cellular immunity to be induced more efficiently. So, the research group focused on cationic lipids, which are known to activate immune cells.
The research group added positively charged cationic lipids to the liposomes, which then increased the amount of negatively charged pH-responsive polysaccharides that could be added to the liposome’s surface. Since dendritic cells—a type of antigen-presenting cell—easily take up negatively charged particles, the uptake of these modified liposomes by the dendritic cells increased by approximately five times, resulting in an approximate 100-time increase in cytokine production.
When the modified liposomes were administered to cancer tissue, M1-type macrophages which help the anticancer immune response increased, while M2-type macrophages which can promote cancer growth decreased. When a vaccine using these liposomes was administered to mice that had been inoculated with cancer cells, a strong anticancer immune response was induced, suppressing cancer growth even though the liposomes only contained one-tenth of the amount of antigen needed in the group’s previous work.
“In the future, we will continue developing antigen carriers that can be used in cancer immunotherapy and vaccines for infectious diseases by combining them with practical antigens,” Professor Yuba concluded.
###
About OMU
Osaka Metropolitan University is a new public university established in April 2022, formed by merger between Osaka City University and Osaka Prefecture University. For more research news visit https://www.upc-osaka.ac.jp/new-univ/en-research/research/ or follow @OsakaMetUniv_en and #OMUScience.