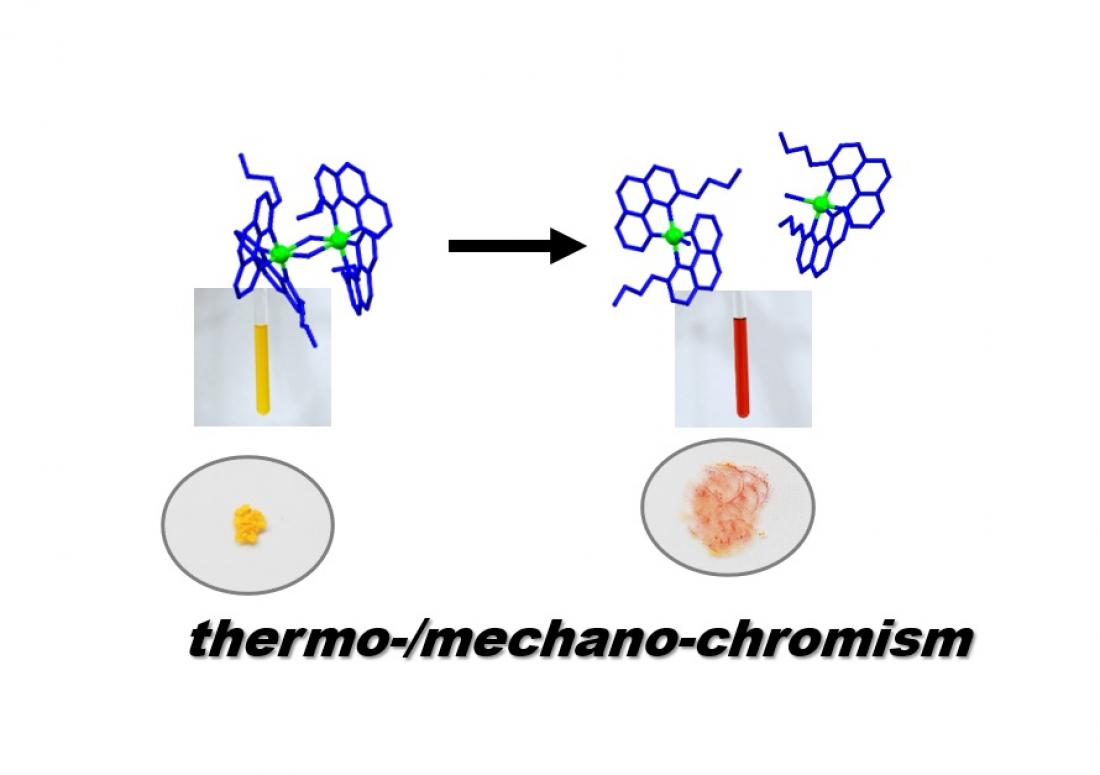
Schematic image of thermochromism and mechanochromism
This paper describes chiral coordination dimers that emerge based on effectively exclusive chiral self-sorting. The complex also exhibits thermo-/mechano-chromism originating from monomer-dimer transformation. The homochiral dimer is comprised of a coordinatively unsaturated iridium(III) complex, which features an n-butyl-substituted benzo[h]quinoline moiety and helical chirality at the metal center. Construction of the appropriate binding model and analysis of the fundamental physical parameters based on spectroscopic data reveal that the strong preference for homochiral dimerization is an entropic-driven effect originating from steric repulsions of alkyl chains in the coordination sphere of the corresponding heterochiral dimer. Furthermore, the metastable nature of dimer crystals allows for color variation (from yellow to red) upon mechanical cleavage of its coordination bonds (i.e., dimer-to-monomer transformation). This feature might be exploited for the dynamic control of coordination geometry and related functionalities, such as catalytic applications. Emergence of strong homochiral self-sorting preference and connected thermo-/mechano-chromic behaviour is based on matched propeller-shaped chirality and subtle steric repulsions of substituents that render particular homochiral dimers switchable and metastable.
This work provides substantial insight into chiral self-sorting in discrete supramolecular systems and its application in the rational design of switchable and metastable dynamic molecular structures with potential as advanced catalysts, sensors, or optoelectronic devices.