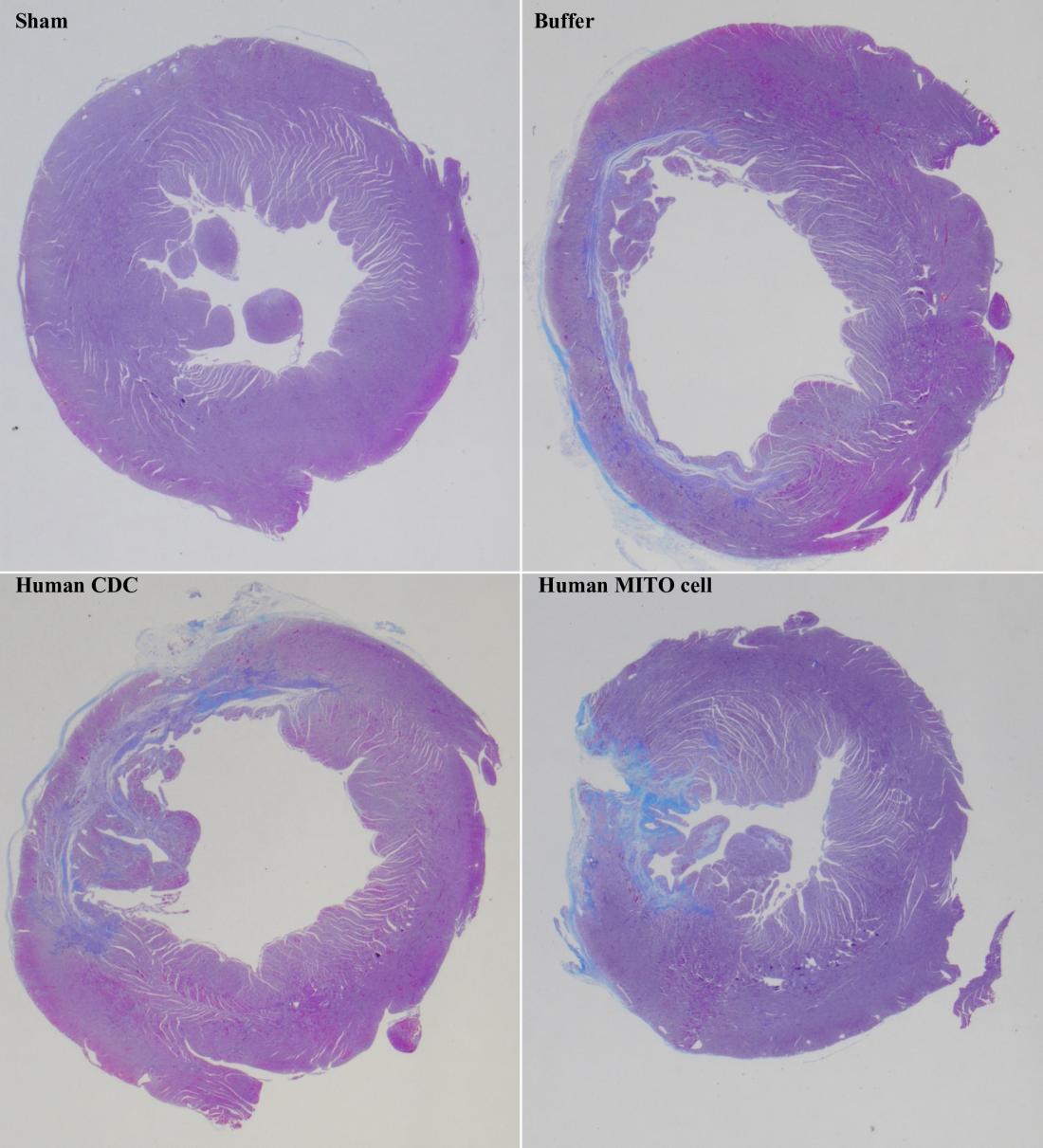
Treatment with human MITO cells (bottom right) effectively suppresses myocardial fibrosis in rat models with myocardial ischemia-reperfusion injury, compared to treatment with human CDCs (bottom left). Sham (top left) represents undamaged cardiac tissue, while Buffer (top right) represents damaged tissue without cell administration. (Masahiro Shiraishi, et al. Journal of Controlled Release. February 3, 2024)
Heart failure stands as a leading cause of mortality worldwide, demanding advanced treatment options. Despite the urgency for more effective treatments, options for severe heart failure remain limited. Cell transplantation therapy has emerged as a promising ray of hope, as it can be used in regenerative therapy to heal the heart.
A research team led by Professor Yuma Yamada of Hokkaido University’s Faculty of Pharmaceutical Science has developed a technique to promote cardiac regeneration by delivering mitochondrial activators to cardiac progenitor cells. Their findings were published in the Journal of Controlled Release.
“Cardiomyocytes efficiently use the mitochondrial tricarboxylic acid cycle to produce large amounts of adenosine triphosphate from several substrates via oxidative phosphorylation (OXPHOS),” explains Yamada. “Based on the energy metabolism of cardiomyocytes, we hypothesized that activating the mitochondrial function of transplanted cells may improve the outcome of cell transplantation therapy.”
Yamada and his group have previously developed a drug delivery system called MITO-Porter, which targets mitochondria within cells. In the current study, they used MITO-Porter to deliver Coenzyme Q10 (CoQ10) to human cardiosphere-derived cells (CDCs), activating their mitochondria (human MITO cells). When these human MITO cells were transplanted into a rat model of myocardial ischemia-reperfusion injury, cardiac function significantly improved. A remarkable ability to suppress myocardial fibrosis was also demonstrated, which could prevent incorrect healing of heart tissue.
Human MITO cells exhibited the ability to improve cardiac function not only through myocardial administration but also with intravenous administration, hinting at versatile therapy applications. The study also suggests that human MITO cells may possess a higher survival rate even in environments characterized by increased Reactive Oxygen Species (ROS), which occurs due to mitochondrial damage.
The researchers employed metabolomics analysis to quantitatively assess metabolic changes in the chronic phase of heart failure in rat models. The study proposes that after myocardial administration of human MITO cells, amino acid synthesis in myocardial TCA cycle in chronic heart failure was enhanced. This suggests that the administration of human MITO cells to the myocardium during the acute phase of myocardial injury may allow the myocardium to effectively utilize the TCA cycle during the chronic phase.
“The strides made in mitochondrial activation bring us closer to a future where cardiac therapy is not just a treatment but a transformative intervention. As we unlock the secrets within our cells, a healthier and more resilient heart stands on the horizon, promising a new dawn in the fight against heart failure,” Yamada concludes.
Atsuhito Takeda (left), Masahiro Shiraishi (center) and Yuma Yamada (right) of the research team (Photo: Yuma Yamada)