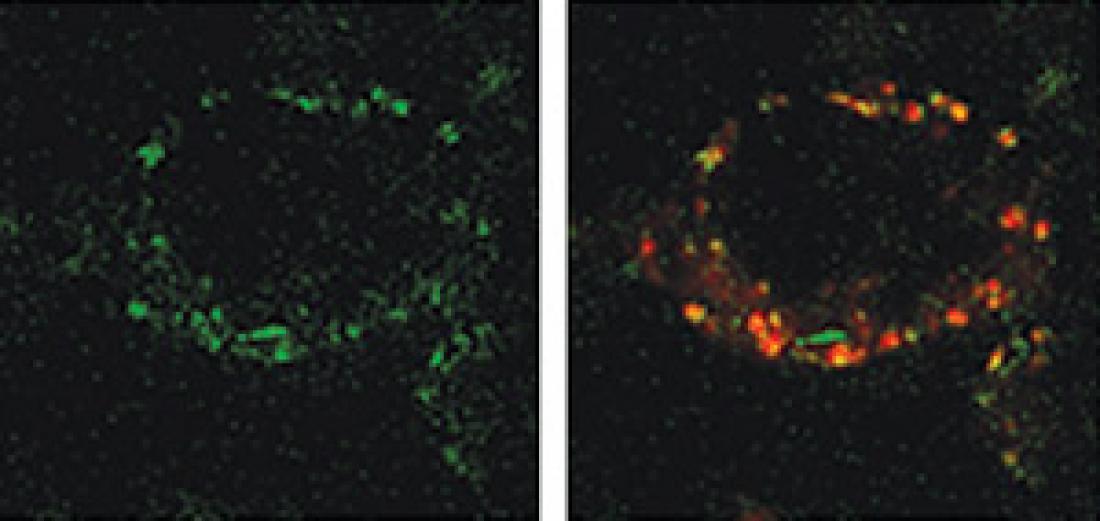
The fusion molecule binds to the misfolded Huntingtin protein (green, left) and recruits the chaperone-mediated autophagy machinery, which sends them to the lysosome (red, right) for degradation.
Huntington’s disease (HD) is a progressive neurodegenerative disease characterized primarily by involuntary movements. Inherited mutations in the huntingtin gene cause a stretch of glutamine residues in its associated protein, huntingtin, to increase in length, so that the mutant protein misfolds and accumulates within neurons. Neurologists believe that failure to clear aggregates of this misfolded protein is an underlying mechanism involved in the onset of HD.
Nerve cells can now be induced to destroy mutant huntingtin protein and reduce aggregate formation, according to the results of a study led by Nobuyuki Nukina of the RIKEN Brain Science Institute in Wako1. The researchers suggest that their approach could be used to effectively treat HD.
Nukina and colleagues used genetic engineering to construct a fusion protein consisting of two copies of polyglutamine binding peptide 1, which is known to bind mutant huntingtin and suppress its aggregation, and the binding regions of heat shock cognate protein 70 (HSC70), which is a ‘chaperone’ protein that targets the mutant huntingtin for destruction.
The researchers found that their construct inhibited the aggregation of mutant huntingtin in cultured cells by inducing a process called chaperone-mediated autophagy, which does not normally break down the misfolded proteins. They also observed that the fusion molecule bound to the mutant huntingtin, forming complexes with HSC70 that were recognized as abnormal and sent to a structure called the lysosome for degradation.
The construct was also effective in clearing aggregates of several other misfolded proteins, including ataxin1, which causes a neurodegenerative disease called spinocerebellar ataxia.
The researchers tested their construct in two strains of mice with HD symptoms. They injected viral vectors containing the construct into the striatum, a brain region that is involved in the control of movement and degenerates in HD patients.
When they examined the animals’ brains four weeks later, they found the construct widely distributed throughout the striatum in both mouse strains. Importantly, the fusion protein had significantly inhibited huntingtin aggregation compared to control mice. As well as reducing the number of aggregates, the construct reduced the average size of the remaining aggregates. The treatment also alleviated HD symptoms in the animals: their movements improved in a behavioral task, they lost less weight, and their survival rate increased.
“Genetic therapy for Huntington's is currently not possible because of the difficulties involved in delivering genes to the brain,” says Nukina, “so we would like to develop or find a compound that can bind to expanded glutamine tracts and HSC70.”
The corresponding author for this highlight is based at the Laboratory for Structural Neuropathology, RIKEN Brain Science Institute