Fig. 1
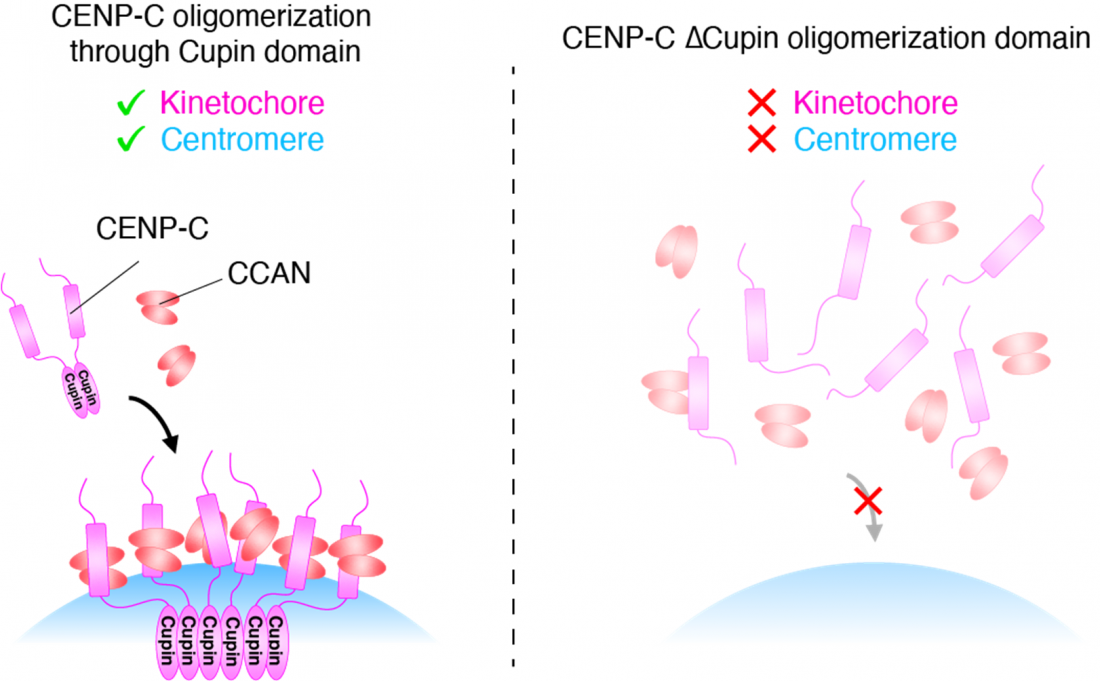
CENP-C oligomerization through Cupin domain is crucial for kinetochore/centromere assembly
A team led by researchers at Osaka University uncover the molecular details of how a crucial protein facilitates proper chromosome movement when our cells divide
Osaka, Japan – During cell division, chromosomes, i.e., molecules containing our genetic material, must be properly replicated and segregated so that each daughter cell receives a complete and accurate set. Now, in an article published in Molecular Cell, a team led by researchers at Osaka University have identified a protein central to this critical process.
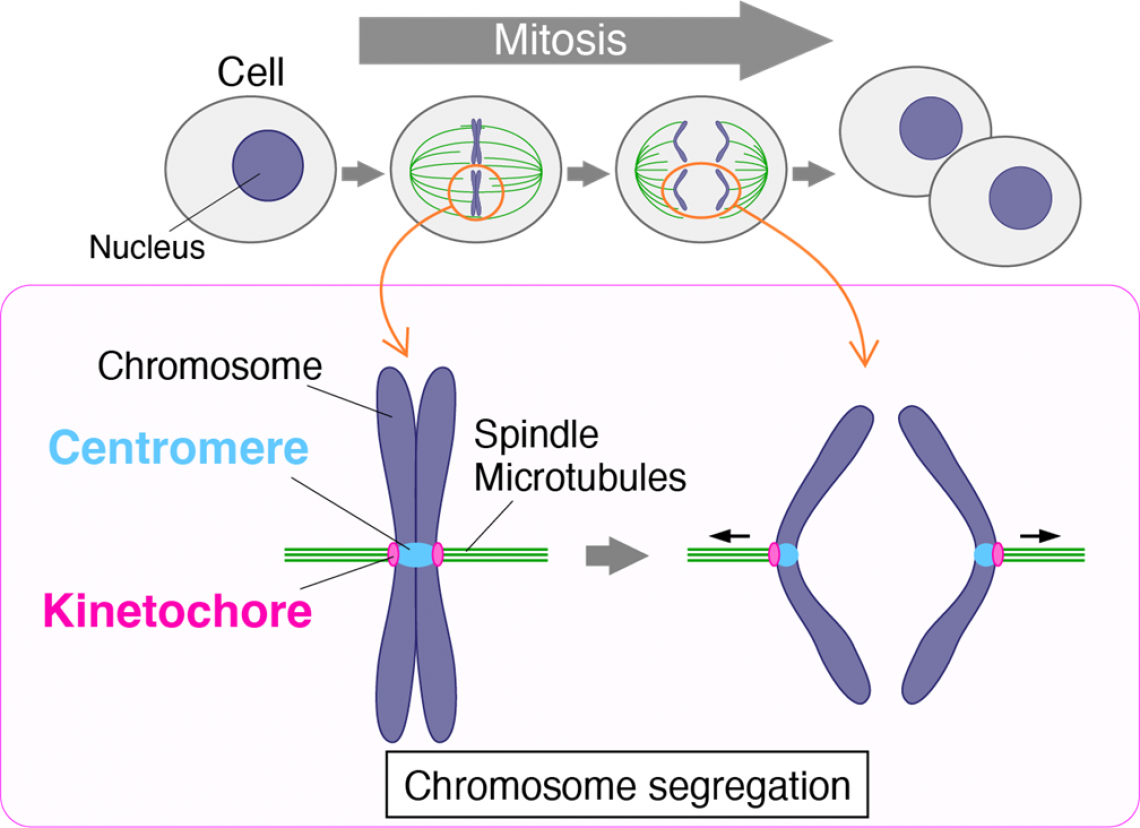
First, some background. Prior to cell division, the two copies of each chromosome are fused together at a region called the centromere. When it’s time to separate, they are pulled away from each other along rope-like microtubules into their respective daughter cells. A protein complex called the kinetochore connects the centromere of each chromosome to its respective microtubules and is thus vital to chromosome segregation.
The ‘constitutive centromere-associated network’ (CCAN), a subcomplex of the kinetochore fixed to the centromere, is an important base upon which the kinetochore can assemble and bind to the microtubules. Previous data suggested that one CCAN protein, CENP-C, is particularly important but its exact role has remained unclear. Therefore, the research team used biochemical analyses to examine how CENP-C contributes to chromosome segregation.
“Though the various species studied in laboratories are very different, such as yeast, chickens, and humans, CENP-C is found in all of them,” says Masatoshi Hara, lead author of the study. “This is called conservation, and it indicates to scientists that this protein has an essential role in cells.”
Fig. 2
Chromosome segregation and kinetochore
The team aimed to determine which portions of the CENP-C protein, called domains, were key to its function. They worked with chicken cells that were engineered so that CENP-C protein expression could be turned off when desired. This allowed researchers to express experimental versions of the CENP-C protein with individual domains deleted and examine the effects on the cells.
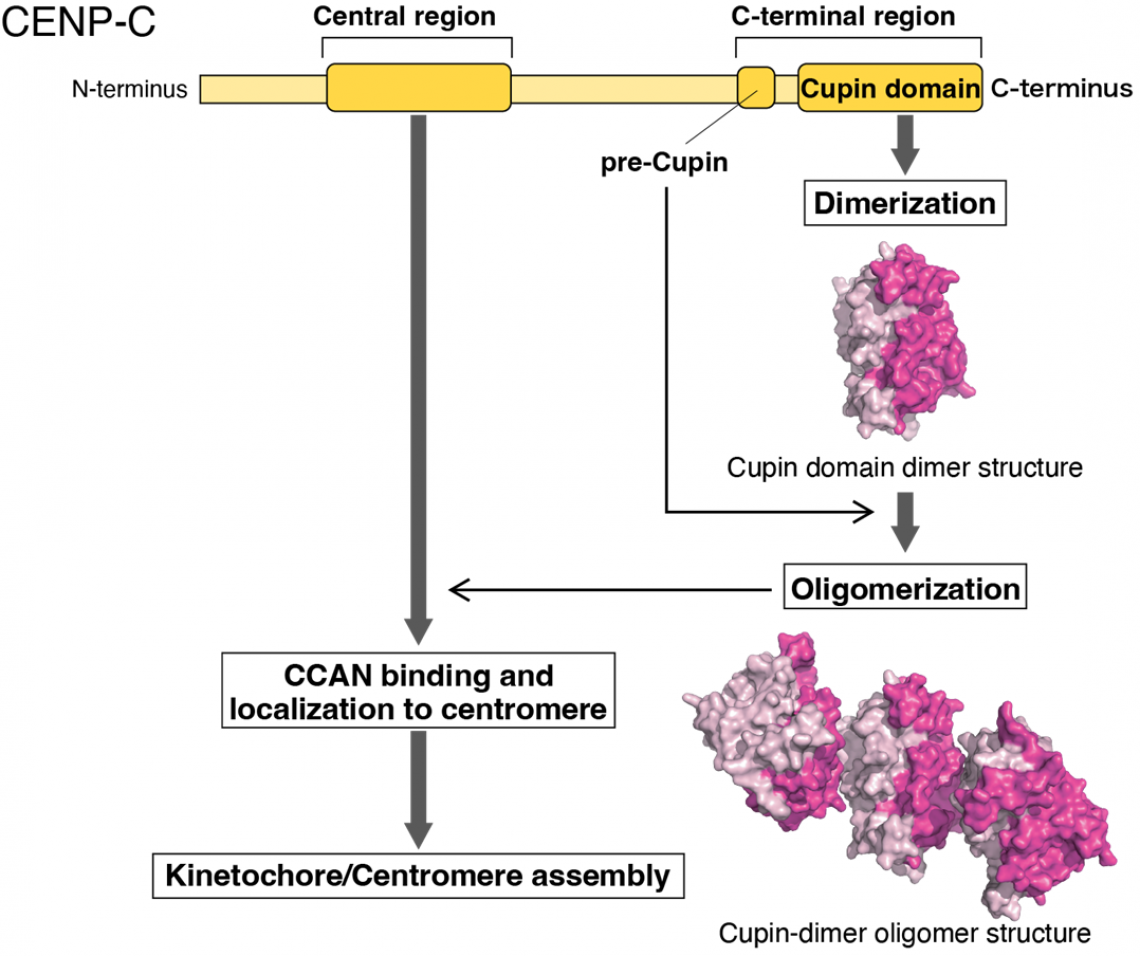
“We deleted two portions of CENP-C: the CCAN-binding domain and the C-terminal region, which contains a domain known as Cupin,” explains Tatsuo Fukagawa, senior author of the article. “Our data show that both are necessary and sufficient for CENP-C to function normally in chicken cells. The cells could not grow and divide properly without them.”
Further experiments demonstrated that the Cupin domain of CENP-C, in both chickens and humans, forms a series of repeating units. In chicken cells with CENP-C expression turned off, experimentally expressing a version of the CENP-C with the Cupin domain deleted could not restore normal growth function to the cells.
“Our analyses indicate that Cupin domain oligomerization is essential for CENP-C to function normally, specifically through supporting CCAN localizing to centromeres and facilitating kinetochore organization,” says Hara.
These findings show us one way in which the body maintains its genomic integrity; such information could help to develop therapeutics for preventing or treating diseases associated with the genome. Furthermore, by revealing that CENP-C supports centromere/kinetochore assembly through the activity of the Cupin domain, this study has uncovered a molecular mechanism underlying one of life’s most fundamental processes.
###
The article, “Centromere/kinetochore is assembled through CENP-C oligomerization,” will be published in Molecular Cell at DOI: https://doi.org/10.1016/j.molcel.2023.05.023
Fig. 3
Oligomerization of CENP-C Cupin domain and its function
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en