Background
Non-alcoholic fatty liver disease (NAFLD)*1) is known as a general term for diseases that cause liver damage due to increased fat accumulation in liver cells (hepatocytes) as a result of excessive intake of nutrients. NAFLD is a chronic liver disease that includes fatty liver and steatohepatitis and progresses to liver cirrhosis and cancer. In addition, in NAFLD, acute liver damage caused by drugs and other factors is exacerbated and prolonged. NAFLD is closely related to acute/chronic liver damage while its prevalence is extremely high, reaching 20-40%. However, effective drug therapy for NAFLD has not yet been established and its development is urgently required.
Liver damage occurs when liver cells "die." In fact, in fatty liver, i.e. hepatic steatosis, the number of hepatocellular deaths increases as the disease becomes more severe. So far, it has been considered that hepatocellular death is caused by a "mode of cell death"*2) called apoptosis and that an increase in apoptosis plays an important role in liver damage. Apoptosis is known as silent cell death without inducing inflammation. In apoptosis, cells condense without disrupting the cell membrane and cell death occurs. Therefore, in apoptosis, various intracellular substances that would cause inflammation are not released and inflammation does not occur. However, in actual fatty liver diseases, hepatitis develops as the disease becomes severe, creating a vicious circle of hepatocellular death and inflammation. In recent years, the involvement of cell death modes other than apoptosis in fatty liver has been proposed but the details have not been elucidated.
Research results
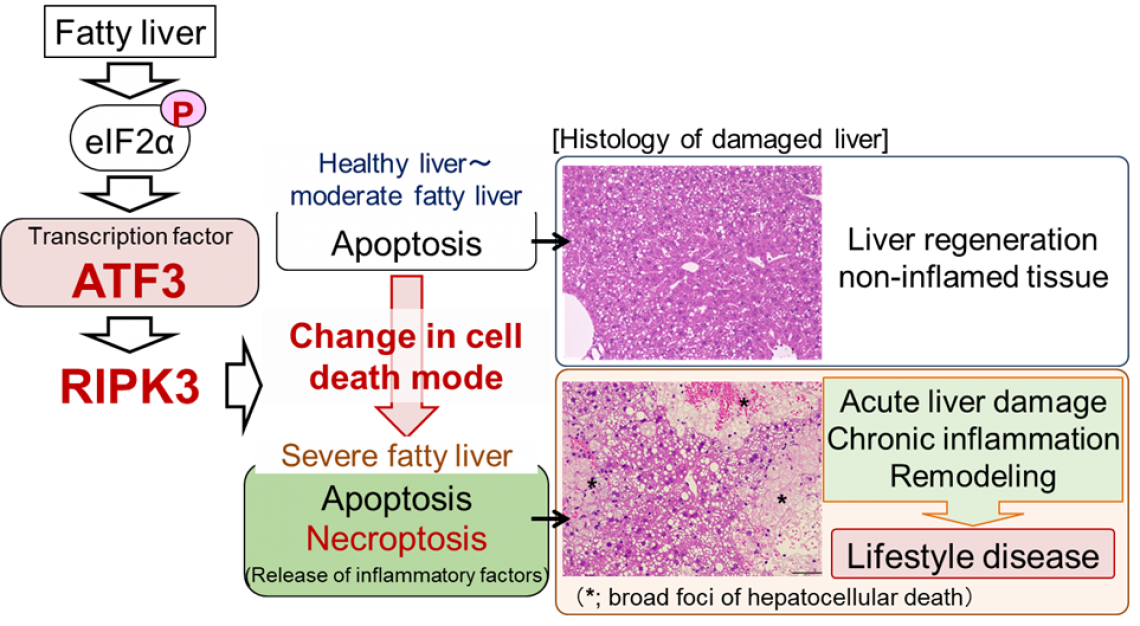
This study was performed by a research team led by Profs. H. Inoue and Y. Inaba, Institute for Frontier Science Initiative, Kanazawa University. In this study, through research using mouse models, we revealed that the cell death mode changes from apoptosis to necroptosis as fatty liver becomes more severe. In necroptosis, cells swell, rupture and die. Therefore, necroptosis causes strong inflammation around dead cells. Although necroptosis is induced by the regulator RIPK3*3), it was considered that necroptosis did not occur in hepatocytes, since they were known to express very little RIPK3. In this study, we found that hepatocytes increased the amount of RIPK3 as a result of the increase in fat accumulation and the cell death mode changed to necroptosis. We also revealed that in the RIPK3-deficient liver, hepatocellular death and liver damage were minimal even with severe fatty liver.
In addition, we discovered that the stress-inducible transcription factor ATF3*4) is a master regulator of necroptosis induction associated with aggravation of hepatic steatosis. So far, we have found that eIF2α signalling*5), an intracellular stress response pathway, is important as a mechanism for inducing hepatocellular death in fatty liver (Hepatology 61, 1343-1356 (2015)). Various intracellular stresses such as oxidative stress and endoplasmic reticulum stress are enhanced in fatty liver and eIF2α signalling is known to be a stress response pathway commonly induced by these intracellular stresses. In this study, we found that stress-inducible transcription factor ATF3, an eIF2α signalling molecule, increases the RIPK3 level and induces necroptosis in severe fatty liver. In fact, ATF3-deficient mouse liver did not undergo necroptosis with increased severity of hepatic steatosis and liver damage was minimal. These findings suggest that hepatic damage due to aggravation of hepatic steatosis is caused by eIF2α signalling/ATF3-mediated RIPK3/necroptosis induction. In this study, we also performed pathological analysis of human steatohepatitis and found that there is a close relationship between hepatocellular damage and the expression levels of ATF3 and RIPK3 in human steatohepatitis cases.
Future prospects
Elucidation of the onset mechanism of acute and chronic liver damage associated with aggravation of hepatic steatosis in this study will lead to understanding of the pathology of NAFLD and development of new treatment methods. In particular, the molecular mechanism of eIF2α signalling/ATF3/RIPK3, which regulates the change in mode of hepatocellular death in fatty liver, is expected to be a novel therapeutic target for NAFLD.
Funder
The Japan Agency for Medical Research and Development (AMED) through AMED-CREST and the Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus; the Japan Science and Technology Agency (JST), CREST; and KAKENHI Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS).
Grant number
JP20gm1210002, AMED-CREST; JP21ek0210156, AMED; JPMJCR2123, JST-CREST; P20H04943, JP20H04102, JP22K19545, JP17H06300, JP19K09192, and JP22H03507, JSPS KAKENHI Grants-in-Aid.
Article
Journal: Nature Communications 14, Article number: 167 (2023)
Title: The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice
Authors: Yuka INABA, Emi HASHIUCHI, Hitoshi WATANABE, Kumi KIMURA, Yu OSHIMA, Kohsuke TSUCHIYA, Shin MURAI, Chiaki TAKAHASHI, Michihiro MATSUMOTO, Shigetaka KITAJIMA, Yasuhiko YAMAMOTO, Masao HONDA, Shun-ichiro ASAHARA, Kim RAVNSKJAER, Shin-ichi HORIKE, Shuichi KANEKO, Masato KASUGA, Hiroyasu NAKANO, Kenichi HARADA, Hiroshi INOUE
https://www.nature.com/articles/s41467-023-35804-w
https://doi.org/10.1038/s41467-023-35804-w
Published online on Jan. 23, 2023
Glossary
*1) Non-alcoholic fatty liver disease (NAFLD)
NAFLD is a general term for liver diseases that develop due to accumulation of fat in the liver in an alcohol-independent manner, including simple fatty liver to non-alcoholic steatohepatitis (NASH) and progression to liver cirrhosis. NASH is accompanied by inflammation and fibrosis and progresses to liver cirrhosis and cancer.
*2) Mode of cell death
Types of cell death classified according to the cell death characteristics and regulatory factors. They are mainly divided into non-inflammatory apoptosis and inflammatory lytic cell death. As for inflammatory lytic cell death, molecularly controlled necroptosis, pyroptosis, ferroptosis, etc. have been reported in addition to necrosis passively induced by external factors.
*3) RIPK3 (receptor interacting protein 3 kinase)
Regulator of necroptosis, one of the inflammatory cell death modes. It is activated by cell death receptors, etc., inducing phosphorylation and multimerization of MLKL, a necroptosis-executing factor, cell membrane disruption and cell death.
*4) Transcription factor ATF3 (activating transcription factor 3)
A transcription factor that is activated in response to various cellular stresses. Involved in the regulation of various cell functions such as cell proliferation, differentiation, and cell death.
*5) eIF2 signalling
eIF2 signalling is a signalling pathway also referred to as an integrated stress response. Signal transduction is induced by phosphorylation of eIF2α by various intracellular stresses. The phosphorylation of eIF2α increases stress-induced transcription factors such as ATF3 and CHOP through the increase in the amount of the transcription factor ATF4 protein, and these transcription factors trigger stress responses.
Figure. Schematic diagram of induction mechanism of acute and chronic liver damage in severe fatty liver
Although mild to moderate fatty liver tends to repair after liver damage, in severe fatty liver, inflammation occurs during the regenerating process after damage and further liver damage is induced. Changes in cell death mode after liver damage play an important role in the pathogenesis. More specifically, the mode of hepatocellular death changes from non-inflammatory apoptosis to inflammatory necroptosis when fatty liver becomes severe. In addition, this change in cell death mode is dependent on the activation of eIF2α signalling/ATF3 associated with aggravation of hepatic steatosis and the accompanying increase in RIPK3 levels.