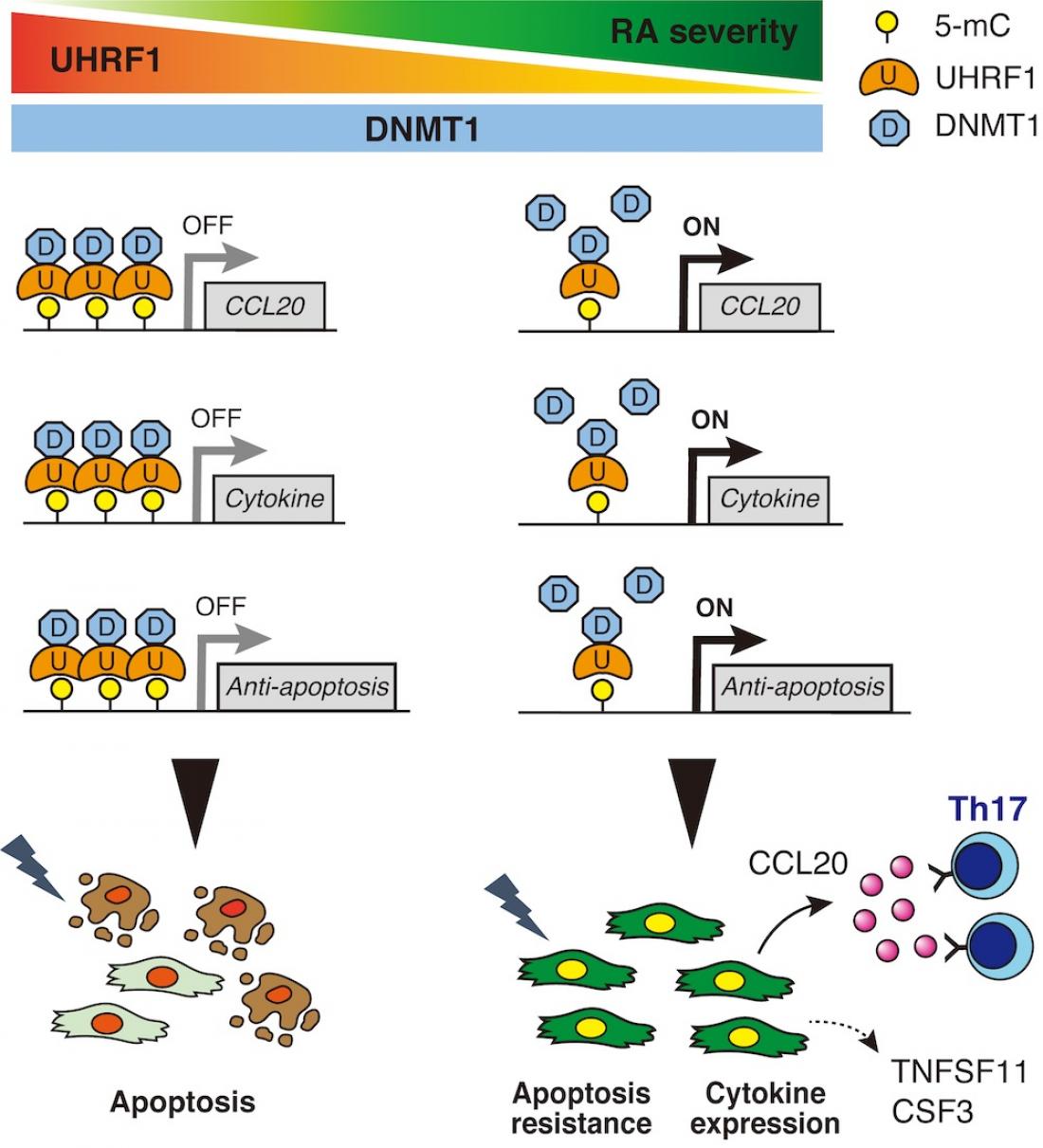
UHRF1 controls DNA methylation of synovial fibroblasts in RA patients.
The UHRF1 expression level is elevated in SF, particularly under RA pathogenesis, but not OA. Sufficient levels of UHRF1 can reduce mRNA expression levels of genes that encode multiple RA-exacerbating factors such as RA-related, cytokine-related and anti-apoptosis-related genes by altering DNA methylation. In contrast, insufficient UHRF1 expression levels are associated with the progression of RA pathogenesis.
Rheumatoid arthritis (RA) is characterized by chronic inflammation of synovium, eventually leading to joint destruction. Epigenetic alteration (the mechanism of gene expression regulation without DNA sequence changes), such as low levels of DNA methylation, is one of the factors which worsens the RA state. However the mechanism by which the alterations occur remains largely unknown.
In the present study, we identified an epigenetic regulator UHRF1 that was remarkably up-regulated in synovial fibroblasts (SF) fromarthritis model mice and RA patients. Previous study showed that UHRF1 is a key player in the maintenance of DNA methylation, although the function for RA is unknown. To understand UHRF1 function for arthritis, we generated mice with SF-specific UHRF1 conditional knockout (cKO) and experimental arthritis was induced. cKO mice exhibited more severe arthritic phenotypes than the littermate control. Next, to reveal UHRF1 function in SF, RNA-seq and MBD-seq were performed using SF obtained from the control and cKO mice. Integrative genome-wide analyses of the transcriptome and methylome showed that expression of several cytokines was up-regulated in UHRF1-deficient SF accompanied by reduced DNA methylation signatures. Also, UHRF1 expression in synovium was negatively correlated with several pathogenesis in RA patients. These data suggested that RA pathogenesis is exacerbated when UHRF1 levels are low in SF. Finally, we assessed whether UHRF1 stabilization contributes to improvement of arthritis pathogenesis. Ryuvidine, which was identified as a candidate chemical compound to the stabilize UHRF1 protein, was administrated in arthritis model mice. The results showed that arthritis pathogenesis was ameliorated by treatment with Ryuvidine. Also, the development of organoids derived from RA-SF was suppressed by Ryuvidine.
This study demonstrated that UHRF1 expressed in SF with RA has a protective role in suppressing multiple pathogenic events in arthritis, suggesting that targeting UHRF1 could be a therapeutic strategy for RA.